Advances and challenges in biosensor-based diagnosis of infectious diseases
- PMID: 24524681
- PMCID: PMC4104499
- DOI: 10.1586/14737159.2014.888313
Advances and challenges in biosensor-based diagnosis of infectious diseases
Abstract
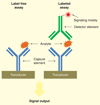
Rapid diagnosis of infectious diseases and timely initiation of appropriate treatment are critical determinants that promote optimal clinical outcomes and general public health. Conventional in vitro diagnostics for infectious diseases are time-consuming and require centralized laboratories, experienced personnel and bulky equipment. Recent advances in biosensor technologies have potential to deliver point-of-care diagnostics that match or surpass conventional standards in regards to time, accuracy and cost. Broadly classified as either label-free or labeled, modern biosensors exploit micro- and nanofabrication technologies and diverse sensing strategies including optical, electrical and mechanical transducers. Despite clinical need, translation of biosensors from research laboratories to clinical applications has remained limited to a few notable examples, such as the glucose sensor. Challenges to be overcome include sample preparation, matrix effects and system integration. We review the advances of biosensors for infectious disease diagnostics and discuss the critical challenges that need to be overcome in order to implement integrated diagnostic biosensors in real world settings.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures



References
-
- Fauci AS, Morens DM. 200 NEJM ANNIVERSARY ARTICLE the perpetual challenge of infectious diseases. N Engl J Med. 2012;366(5):454–461. - PubMed
-
-
Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–144. • Overview of point-of-care diagnostic technologies for global health issues
-
-
- Mabey D, Peeling RW, Ustianowski A, Perkins MD. Diagnostics for the developing world. Nat Rev Microbiol. 2004;2(3):231–240. - PubMed
-
- Luong JH, Male KB, Glennon JD. Biosensor technology: technology push versus market pull. Biotechnol Adv. 2008;26(5):492–500. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
