Chronic murine toxoplasmosis is defined by subtle changes in neuronal connectivity
- PMID: 24524910
- PMCID: PMC3974456
- DOI: 10.1242/dmm.014183
Chronic murine toxoplasmosis is defined by subtle changes in neuronal connectivity
Abstract
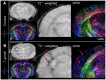
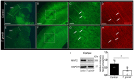
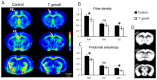
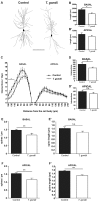
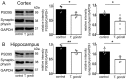
Recent studies correlate chronic Toxoplasma gondii (T. gondii) infection with behavioral changes in rodents; additionally, seropositivity in humans is reported to be associated with behavioral and neuropsychiatric diseases. In this study we investigated whether the described behavioral changes in a murine model of chronic toxoplasmosis are associated with changes in synaptic plasticity and brain neuronal circuitry. In mice chronically infected with T. gondii, magnetic resonance imaging (MRI) data analysis displayed the presence of heterogeneous lesions scattered throughout all brain areas. However, a higher density of lesions was observed within specific regions such as the somatosensory cortex (SSC). Further histopathological examination of these brain areas indicated the presence of activated resident glia and recruited immune cells accompanied by limited alterations of neuronal viability. In vivo diffusion-tensor MRI analysis of neuronal fiber density within the infected regions revealed connectivity abnormalities in the SSC. Altered fiber density was confirmed by morphological analysis of individual, pyramidal and granule neurons, showing a reduction in dendritic arbor and spine density within the SSC, as well as in the hippocampus. Evaluation of synapse efficacy revealed diminished levels of two key synaptic proteins, PSD95 and synaptophysin, within the same brain areas, indicating deficits in functionality of the synaptic neurotransmission in infected mice. Our results demonstrate that persistent T. gondii infection in a murine model results in synaptic deficits within brain structures leading to disturbances in the morphology of noninfected neurons and modified brain connectivity, suggesting a potential explanation for the behavioral and neuropsychiatric alterations.
Keywords: Behavioral manipulation; Neuronal connectivity; Parasites.
Figures

References
-
- Al-Chalabi A, Miller C. C. (2003). Neurofilaments and neurological disease. Bioessays 25, 346–355 - PubMed
-
- Arling T. A., Yolken R. H., Lapidus M., Langenberg P., Dickerson F. B., Zimmerman S. A., Balis T., Cabassa J. A., Scrandis D. A., Tonelli L. H., et al. (2009). Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J. Nerv. Ment. Dis. 197, 905–908 - PubMed
-
- Berdoy M., Webster J. P., Macdonald D. W. (1995). Parasite-altered behaviour: is the effect of Toxoplasma gondii on Rattus norvegicus specific? Parasitology 111, 403–409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

