Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans
- PMID: 24525020
- PMCID: PMC3962593
- DOI: 10.1152/ajpgi.00372.2013
Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans
Abstract
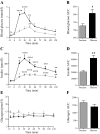
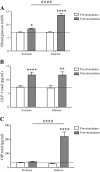
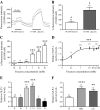
Nutrients often stimulate gut hormone secretion, but the effects of fructose are incompletely understood. We studied the effects of fructose on a number of gut hormones with particular focus on glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). In healthy humans, fructose intake caused a rise in blood glucose and plasma insulin and GLP-1, albeit to a lower degree than isocaloric glucose. Cholecystokinin secretion was stimulated similarly by both carbohydrates, but neither peptide YY3-36 nor glucagon secretion was affected by either treatment. Remarkably, while glucose potently stimulated GIP release, fructose was without effect. Similar patterns were found in the mouse and rat, with both fructose and glucose stimulating GLP-1 secretion, whereas only glucose caused GIP secretion. In GLUTag cells, a murine cell line used as model for L cells, fructose was metabolized and stimulated GLP-1 secretion dose-dependently (EC50 = 0.155 mM) by ATP-sensitive potassium channel closure and cell depolarization. Because fructose elicits GLP-1 secretion without simultaneous release of glucagonotropic GIP, the pathways underlying fructose-stimulated GLP-1 release might be useful targets for type 2 diabetes mellitus and obesity drug development.
Keywords: enteroendocrine axis; gastric inhibitory peptide; glucagon-like peptide-1.
Figures


References
-
- Bismut H, Hers HG, Van Schaftingen E. Conversion of fructose to glucose in the rabbit small intestine. A reappraisal of the direct pathway. Eur J Biochem 213: 721–726, 1993 - PubMed
-
- Bray GA. How bad is fructose? Am J Clin Nutr 86: 895–896, 2007 - PubMed
-
- Curry DL. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas 4: 2–9, 1989 - PubMed
-
- Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol 8: 1646–1655, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

