Pathogens hijack the epigenome: a new twist on host-pathogen interactions
- PMID: 24525150
- PMCID: PMC3970002
- DOI: 10.1016/j.ajpath.2013.12.022
Pathogens hijack the epigenome: a new twist on host-pathogen interactions
Abstract
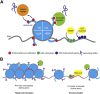
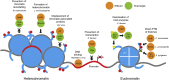
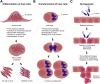
Pathogens have evolved strategies to promote their survival by dramatically modifying the transcriptional profile and protein content of the host cells they infect. Modifications of the host transcriptome and proteome are mediated by pathogen-encoded effector molecules that modulate host cells through a variety of different mechanisms. Recent studies highlight the importance of the host chromatin and other epigenetic regulators as targets of pathogens. Host gene regulatory mechanisms may be targeted through cytoplasmic signaling, directly by pathogen effector proteins, and possibly by pathogen RNA. Although many of these changes are short-lived and persist only during the course of infection, several studies indicate that pathogens are able to induce long-term, heritable changes that are essential to pathogenesis of infectious diseases and persistence of pathogens within their hosts. In this review, we discuss how pathogens modulate the epigenome of host cells, a new and flourishing avenue of host-pathogen interaction studies.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Brodsky I.E., Medzhitov R. Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol. 2009;11:521–526. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

