Regulation of autophagy and mitophagy by nutrient availability and acetylation
- PMID: 24525425
- PMCID: PMC3969632
- DOI: 10.1016/j.bbalip.2014.02.001
Regulation of autophagy and mitophagy by nutrient availability and acetylation
Abstract
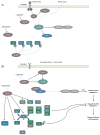
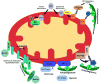
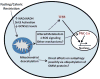
Normal cellular function is dependent on a number of highly regulated homeostatic mechanisms, which act in concert to maintain conditions suitable for life. During periods of nutritional deficit, cells initiate a number of recycling programs which break down complex intracellular structures, thus allowing them to utilize the energy stored within. These recycling systems, broadly named "autophagy", enable the cell to maintain the flow of nutritional substrates until they can be replenished from external sources. Recent research has shown that a number of regulatory components of the autophagy program are controlled by lysine acetylation. Lysine acetylation is a reversible post-translational modification that can alter the activity of enzymes in a number of cellular compartments. Strikingly, the main substrate for this modification is a product of cellular energy metabolism: acetyl-CoA. This suggests a direct and intricate link between fuel metabolites and the systems which regulate nutritional homeostasis. In this review, we examine how acetylation regulates the systems that control cellular autophagy, and how global protein acetylation status may act as a trigger for recycling of cellular components in a nutrient-dependent fashion. In particular, we focus on how acetylation may control the degradation and turnover of mitochondria, the major source of fuel-derived acetyl-CoA.
Keywords: Acetylation; Autophagy; GCN5L1; Mitophagy; Sirt3.
Published by Elsevier B.V.
Figures
References
-
- Altun G, Akansu B, Altun BU, Azmak D, Yilmaz A. Deaths due to hunger strike: post-mortem findings. Forensic Sci Int. 2004;146:35–38. - PubMed
-
- De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. - PubMed
-
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. - PubMed
-
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. - PubMed
-
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

