Targeting CDC25C, PLK1 and CHEK1 to overcome Docetaxel resistance induced by loss of LZTS1 in prostate cancer
- PMID: 24525428
- PMCID: PMC3996665
- DOI: 10.18632/oncotarget.1574
Targeting CDC25C, PLK1 and CHEK1 to overcome Docetaxel resistance induced by loss of LZTS1 in prostate cancer
Abstract
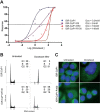
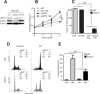
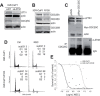
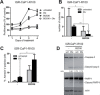
Docetaxel is used as a standard treatment in patients with metastatic castration-resistant prostate cancer. However, a large subset of patients develops resistance. Understanding resistance mechanisms, which are largely unknown, will allow identification of predictive biomarkers and therapeutic targets. We established resistant IGR-CaP1 prostate cancer cell lines for different doses of Docetaxel. We investigated gene expression profiles by microarray analyses in these cell lines and generated a signature of 99 highly differentially expressed genes potentially implicated in chemoresistance. We focused on the role of the cell cycle regulator LZTS1, which was under-expressed in the Docetaxel-resistant cell lines, its inhibition resulting from the promoter methylation. Knockdown of LZTS1 in parental cells with siRNA showed that LZTS1 plays a role in the acquisition of the resistant phenotype. Furthermore, we observed that targeting CDC25C, a partner of LZTS1, with the NSC663284 inhibitor specifically killed the Docetaxel-resistant cells. To further investigate the role of CDC25C, we used inhibitors of the mitotic kinases that regulate CDC25C. Inhibition of CHEK1 and PLK1 induced growth arrest and cell death in the resistant cells. Our findings identify an important role of LZTS1 through its regulation of CDC25C in Docetaxel resistance in prostate cancer and suggest that CDC25C, or the mitotic kinases CHEK1 and PLK1, could be efficient therapeutic targets to overcome Docetaxel resistance.
Figures


References
-
- Petrylak DP, Tangen CM, Hussain MHA, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. - PubMed
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. - PubMed
-
- Mackler NJ, Pienta KJ. Drug insight: Use of docetaxel in prostate and urothelial cancers. Nat Clin Pract Urol. 2005;2(2):92–100. - PubMed
-
- McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785(2):96–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

