Botulinum neurotoxin A complex recognizes host carbohydrates through its hemagglutinin component
- PMID: 24525478
- PMCID: PMC3942755
- DOI: 10.3390/toxins6020624
Botulinum neurotoxin A complex recognizes host carbohydrates through its hemagglutinin component
Abstract
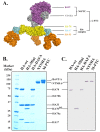
Botulinum neurotoxins (BoNTs) are potent bacterial toxins. The high oral toxicity of BoNTs is largely attributed to the progenitor toxin complex (PTC), which is assembled from BoNT and nontoxic neurotoxin-associated proteins (NAPs) that are produced together with BoNT in bacteria. Here, we performed ex vivo studies to examine binding of the highly homogeneous recombinant NAPs to mouse small intestine. We also carried out the first comprehensive glycan array screening with the hemagglutinin (HA) component of NAPs. Our data confirmed that intestinal binding of the PTC is partly mediated by the HA moiety through multivalent interactions between HA and host carbohydrates. The specific HA-carbohydrate recognition could be inhibited by receptor-mimicking saccharides.
Figures

Similar articles
-
Inhibiting oral intoxication of botulinum neurotoxin A complex by carbohydrate receptor mimics.Toxicon. 2015 Dec 1;107(Pt A):43-9. doi: 10.1016/j.toxicon.2015.08.003. Epub 2015 Aug 10. Toxicon. 2015. PMID: 26272706 Free PMC article.
-
Characterization of Hemagglutinin Negative Botulinum Progenitor Toxins.Toxins (Basel). 2017 Jun 15;9(6):193. doi: 10.3390/toxins9060193. Toxins (Basel). 2017. PMID: 28617306 Free PMC article.
-
High-resolution crystal structure of HA33 of botulinum neurotoxin type B progenitor toxin complex.Biochem Biophys Res Commun. 2014 Apr 4;446(2):568-73. doi: 10.1016/j.bbrc.2014.03.008. Epub 2014 Mar 12. Biochem Biophys Res Commun. 2014. PMID: 24631690 Free PMC article.
-
Botulinum Hemagglutinin: Critical Protein for Adhesion and Absorption of Neurotoxin Complex in Host Intestine.Methods Mol Biol. 2020;2132:183-190. doi: 10.1007/978-1-0716-0430-4_19. Methods Mol Biol. 2020. PMID: 32306327 Review.
-
Assembly and function of the botulinum neurotoxin progenitor complex.Curr Top Microbiol Immunol. 2013;364:21-44. doi: 10.1007/978-3-642-33570-9_2. Curr Top Microbiol Immunol. 2013. PMID: 23239347 Free PMC article. Review.
Cited by
-
Translocation of botulinum neurotoxin serotype A and associated proteins across the intestinal epithelia.Cell Microbiol. 2015 Aug;17(8):1133-43. doi: 10.1111/cmi.12424. Epub 2015 Feb 15. Cell Microbiol. 2015. PMID: 25640773 Free PMC article.
-
Botulinum Toxin as a Pain Killer: Players and Actions in Antinociception.Toxins (Basel). 2015 Jun 30;7(7):2435-53. doi: 10.3390/toxins7072435. Toxins (Basel). 2015. PMID: 26134255 Free PMC article. Review.
-
Chinese botulinum toxin A for the treatment of lower urinary tract dysfunction: It works just as well.Bladder (San Franc). 2022 Sep 14;9(1):e47. doi: 10.14440/bladder.2022.847. eCollection 2022. Bladder (San Franc). 2022. PMID: 36425077 Free PMC article. Review.
-
Sensory Symptoms Associated with Aesthetic Botulinum Toxin A Treatments.Plast Reconstr Surg Glob Open. 2022 Nov 16;10(11):e4631. doi: 10.1097/GOX.0000000000004631. eCollection 2022 Nov. Plast Reconstr Surg Glob Open. 2022. PMID: 36405048 Free PMC article.
-
The hypothetical protein P47 of Clostridium botulinum E1 strain Beluga has a structural topology similar to bactericidal/permeability-increasing protein.Toxicon. 2018 Jun 1;147:19-26. doi: 10.1016/j.toxicon.2017.10.012. Epub 2017 Oct 16. Toxicon. 2018. PMID: 29042313 Free PMC article.
References
-
- Schiavo G., Matteoli M., Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000;80:717–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

