Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy : preparation for gene therapy clinical trial
- PMID: 24525545
- PMCID: PMC4266137
- DOI: 10.1001/jamaophthalmol.2013.7971
Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy : preparation for gene therapy clinical trial
Abstract
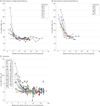
IMPORTANCE Establishing the natural history of G11778A Leber hereditary optic neuropathy (LHON) is important to determine the optimal end points to assess the safety and efficacy of a planned gene therapy trial. OBJECTIVE To use the results of the present natural history study of patients with G11778A LHON to plan a gene therapy clinical trial that will use allotopic expression by delivering a normal nuclear-encoded ND4 gene into the nuclei of retinal ganglion cells via an adeno-associated virus vector injected into the vitreous. DESIGN, SETTING, AND PARTICIPANTS A prospective observational study initiated in 2008 was conducted in primary and referral institutional practice settings. Participants included 44 individuals with G11778A LHON, recruited between September 2008 and March 2012, who were evaluated every 6 months and returned for 1 or more follow-up visits (6-36 months) as of August 2012. EXPOSURES Complete neuro-ophthalmic examination and main measures. MAIN OUTCOMES AND MEASURES Visual acuity, automated visual field testing, pattern electroretinogram, and spectral-domain optical coherence tomography. RESULTS Clinical measures were stable during the follow-up period, and visual acuity was as good as or better than the other visual factors used for monitoring patients. Based on a criterion of 15 or more letters from the Early Treatment Diabetic Retinopathy Study chart, 13 eyes of 8 patients (18%) improved, but 24 months after the onset of symptoms, any further improvements were to no better than 20/100. Acuity recovery occurred in some patients despite continued marked retinal nerve fiber layer thinning indistinguishable from that in patients who did not recover visual acuity. CONCLUSIONS AND RELEVANCE Spontaneous improvement of visual acuity in patients with G11778A LHON is not common and is partial and limited when it occurs, so improvements in vision with adeno-associated virus-mediated gene therapy of a synthetic wild-type ND4 subunit gene should be possible to detect with a reasonable sample size. Visual acuity appears to be the most suitable primary end point for the planned clinical trial.
Conflict of interest statement
Figures


References
-
- Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(pt 2):319–337. - PubMed
-
- Sadun AA, La Morgia C, Carelli V. Leber’s hereditary optic neuropathy. Curr Treat Options Neurol. 2011;13(1):109–117. - PubMed
-
- Bi R, Zhang AM, Yu D, Chen D, Yao YG. Screening the three LHON primary mutations in the general Chinese population by using an optimized multiplex allele-specific PCR. Clin Chim Acta. 2010;411(21–22):1671–1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

