Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species
- PMID: 24525732
- PMCID: PMC3944250
- DOI: 10.1038/cddis.2014.33
Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species
Abstract
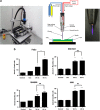
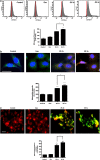
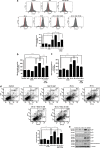
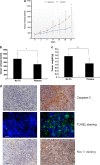
Nonthermal plasma (NTP) is generated by ionization of neutral gas molecules, which results in a mixture of energy particles including electrons and ions. Recent progress in the understanding of NTP has led to its application in the treatment of various diseases, including cancer. However, the molecular mechanisms of NTP-induced cell death are unclear. The purpose of this study was to evaluate the molecular mechanism of NTP in the induction of apoptosis of head and neck cancer (HNC) cells. The effects of NTP on apoptosis were investigated using MTT, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling, Annexin V assays, and western blot analysis. The cells were examined for production of reactive oxygen species (ROS) using DCFCA or MitoSOX staining, intracellular signaling, and an animal model. NTP reduced HNC cell viability in a dose-dependent manner and induced apoptosis. NTP resulted in alteration of mitochondrial membrane potential and accumulation of intracellular ROS generated from the mitochondria in HNC cells. Blockade of ROS production by N-acetyl-L-cysteine inhibited NTP-induced apoptosis. NTP led to the phosphorylation of c-JUN N-terminal kinase (JNK) and p38, but not extracellular-regulated kinase. Treatment with JNK and p38 inhibitors alleviated NTP-induced apoptosis via ROS generation. Taken together, these results show that NTP induced apoptosis of HNC cells by a mechanism involving MAPK-dependent mitochondrial ROS. NTP inhibited the growth of pre-established FaDu tumors in a nude mouse xenograft model and resulted in accumulation of intracellular ROS. In conclusion, NTP induced apoptosis in HNC cells through a novel mechanism involving MAPK-mediated mitochondrial ROS. These findings show the therapeutic potential of NTP in HNC.
Figures



References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. Cancer J Clin. 2009;59:225–249. - PubMed
-
- Kim CH, Bahn JH, Lee SH, Kim GY, Jun SI, Lee K, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010;150:530–538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

