Detection of brain tumor cells in the peripheral blood by a telomerase promoter-based assay
- PMID: 24525740
- PMCID: PMC4144786
- DOI: 10.1158/0008-5472.CAN-13-0813
Detection of brain tumor cells in the peripheral blood by a telomerase promoter-based assay
Abstract
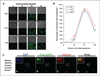
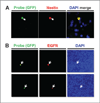
Blood tests to detect circulating tumor cells (CTC) offer great potential to monitor disease status, gauge prognosis, and guide treatment decisions for patients with cancer. For patients with brain tumors, such as aggressive glioblastoma multiforme, CTC assays are needed that do not rely on expression of cancer cell surface biomarkers like epithelial cell adhesion molecules that brain tumors tend to lack. Here, we describe a strategy to detect CTC based on telomerase activity, which is elevated in nearly all tumor cells but not normal cells. This strategy uses an adenoviral detection system that is shown to successfully detect CTC in patients with brain tumors. Clinical data suggest that this assay might assist interpretation of treatment response in patients receiving radiotherapy, for example, to differentiate pseudoprogression from true tumor progression. These results support further development of this assay as a generalized method to detect CTC in patients with cancer.
©2014 AACR.
Conflict of interest statement
The coauthors are employees of the University of Pennsylvania, which has a patent pending on components of the technology described in this manuscript. J.F. Dorsey, G.D. Kao, and S.M. Hahn are cofounders of Liquid Biotech LLC (Penn CTT/ UpSTART). No potential conflicts of interest were disclosed by the other authors.
Figures


References
-
- Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

