Automated weaning and spontaneous breathing trial systems versus non-automated weaning strategies for discontinuation time in invasively ventilated postoperative adults
- PMID: 24526330
- PMCID: PMC6517122
- DOI: 10.1002/14651858.CD008639.pub2
Automated weaning and spontaneous breathing trial systems versus non-automated weaning strategies for discontinuation time in invasively ventilated postoperative adults
Abstract
Background: Automated systems use closed-loop control to enable ventilators to perform basic and advanced functions while supporting respiration. Selected automated systems can now not only measure selected respiratory variables and adapt ventilator output to individual patient needs by operationalizing predetermined algorithms but also automate the conduct of spontaneous breathing trials (SBTs).
Objectives: To summarize the evidence comparing automated weaning and SBT systems to non-automated mechanical ventilation strategies on time to mechanical ventilation discontinuation in adult postoperative patients. In secondary objectives we ascertained differences between automated weaning and SBT systems and non-automated mechanical ventilation discontinuation strategies on clinical outcomes (time to successful extubation, time to first SBT and first successful SBT, mortality, total duration of ventilation, intensive care unit (ICU) and hospital lengths of stay, use of non-invasive ventilation (NIV) following extubation, and adverse events).
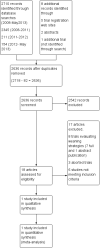
Search methods: We searched CENTRAL (The Cochrane Library 2013, Issue 5); MEDLINE (OvidSP) (1966 to May 2013); EMBASE (OvidSP) (1988 to May 2013); CINAHL (EBSCOhost) (1982 to May 2013), Evidence Based Medicine Reviews and Ovid Health Star (1999 to May 2013), conference proceedings, trial registration websites, and contacted authors and content experts to identify potentially eligible trials.
Selection criteria: Randomized and quasi-randomized trials comparing automated weaning and SBT systems to non-automated mechanical ventilation discontinuation strategies in intubated adults in the postoperative setting.
Data collection and analysis: Two review authors independently assessed trial quality and abstracted data according to prespecified criteria. Sensitivity and subgroup analyses were planned to assess the impact of the type of (i) clinician primarily involved in implementing the automated weaning and SBT systems, (ii) intensive care unit (ICU), and (iii) non-automated discontinuation (control) strategy utilized on selected outcomes.
Main results: We identified one randomized controlled trial of high quality, involving 300 patients , comparing SmartCare™ to a written protocol. In this trial, SmartCare™ had no effect on discontinuation time. While SmartCare™ significantly reduced the time to the first SBT (mean difference (MD) -0.34 days, 95% CI -0.60 to -0.08; P = 0.01) it did not reduce the time to the first successful SBT (MD -0.25 days, 95% CI -0.55 to 0.05; P = 0.10) and other clinically important outcomes. SmartCare™ did not demonstrate beneficial effects on most clinically important outcomes including time to successful extubation, total duration of mechanical ventilation, ICU and hospital lengths of stay, and the requirement for tracheostomy. Moreover, SmartCare™ did not favourably impact reintubation, mortality, self-extubation, and the proportion of patients undergoing protracted mechanical ventilation, with a small numbers of events in this single trial.
Authors' conclusions: There is a paucity of evidence from randomized controlled trials to support or refute use of automated weaning and SBT systems in discontinuing invasive mechanical ventilation in adult postoperative patients. In a single large trial of high methodologic quality, while the use of SmartCare™ to adjust ventilator settings and conduct SBTs shortened the time to undergoing the first SBT, it did not reduce the time to the first successful SBT or the rate of tracheostomy compared to a written protocol implemented by physicians. SmartCare™ did not demonstrate beneficial effects on clinically important outcomes including time to mechanical ventilation discontinuation, time to successful discontinuation, total duration of mechanical ventilation, and ICU and hospital lengths of stay. Additional well-designed, adequately powered randomized controlled trials are needed to clarify the role for SmartCare™ on important outcomes in patients who predominantly require short term ventilation and in specific postoperative patient populations.
Conflict of interest statement
Drs Burns and Lellouche hold a $5000 CDN travel bursary from Draeger Medical Inc (Canada) for the purpose of conducting site visits to participating centres in the WEAN Study. The WEAN Study is an investigator‐initiated trial comparing SmartCare™ and protocolized weaning for which the co‐principal investigators (Drs Burns and Lellouche) obtained peer‐review funding to implement. Draeger Medical Inc provided ventilators and ventilator upgrades for the WEAN Study and a central randomization system using electronic mail correspondence (Draeger Medical, Germany). Draeger Medical was not involved in any aspects of the study design and oversight, data management or data analysis.
Drs Burns, Lellouche and Lessard have self‐identified as investigators of trials that apply the intervention in question. However, the methods used in conducting this review do not allow bias in the selection, data extraction or risk of bias assessment of any included or excluded studies from these authors.
Drs Friedrich and Nisenbaum have no conflicts of interest to declare.
Figures


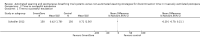











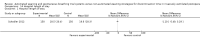
Update of
- doi: 10.1002/14651858.CD008639
References
References to studies included in this review
Schadler 2012 {published and unpublished data}
-
- Schadler D, Engel C, Gunnar E, Pulletz S, Haake N, Frerichs I, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. American Journal of Respiratory and Critical Care Medicine 2012;185(6):637‐44. [PUBMED: 22268137] - PubMed
References to studies excluded from this review
Beale 2007 {unpublished data only}
-
- Beale R. Comparison of an automated weaning programme and a standard clinical weaning protocol for weaning critically ill patients. www.controlled‐trials.com 2007. [ISRCTN82559457]
Bifulco 2008 {published data only}
-
- Bifulco F, Donato L, Giuricin F, Narni Mancinelli V, Vicchio C, Servillo G. Weaning with SmartCare: Our experience. 21st ESICM Annual Congress. 2008:S79.
Burns 2012 {unpublished data only}
-
- Burns KEA, Meade MO, Lessard M, Hand L, Keenan SP, Zhou Q, et al. Wean Earlier and Automatically with New Technology: The WEAN Study. American Journal of Respiratory and Critical Care Medicine 2011;183:A6248. - PubMed
Chen 2008 {published data only}
-
- Chen CW, Wu CP, Dai YL, Perng WC, Huang YC. Adaptive support ventilation (ASV) facilitates ventilator weaning in a medical ICU with limited respiratory therapist support. American Journal of Respiratory and Critical Care Medicine 2008;177:A379.
Donglemans 2007 {published data only}
-
- Dongelmans DA, Veelo DP, Binnekade JM, Vroom MB, Schultz MJ. Pressure controlled/pressure support (PC/PS) versus adaptive support ventilation (ASV) in post‐cardiac surgery patients ‐ A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2007;175:A433.
Jiang 2006 {published data only}
-
- Jiang H, Yu S, Wang L. Comparison of SmartCare and spontaneous breathing trials for weaning old patients with chronic obstructive pulmonary disease. Chinese Journal of Tuberculosis and Respiratory Diseases 2006;29:545‐8. [PUBMED: 17074269] - PubMed
Jolliet 2006 {published data only}
-
- Jolliet P, Battisti A, Roeseler J, Tasseaux D. Automatic adjustment of pressure support (PS) with a computer‐driven knowledge‐based system (SmartCare) during noninvasive ventilation (NIV) in acute respiratory failure. Proceedings of the American Thoracic Society 2006;3:A472. - PubMed
Jouvet 2007 {published data only}
-
- Jouvet P, Farges C, Hatzakis G, Monir A, Lesage F, Dupic L, et al. Weaning children from mechanical ventilation with a computer‐driven system (closed‐loop protocol): a pilot study. Pediatric Critical Care Medicine 2007;8:425‐32. [PUBMED: 17693913] - PubMed
Kataoka 2007 {published data only}
-
- Katoako G, Murai N, Kodera K, Sasaki A, Asano R, Ikeda M, et al. Clinical experience with Smart Care after off‐pump coronary artery bypass for early extubation. Journal of Artificial Organs 2007;10:218‐222. [PUBMED: 18071851] - PubMed
Lellouche 2006 {published data only}
Lim 2012 {published and unpublished data}
-
- Lim ETS, Hamid N, Beh NC, Phua GC, Tan J. Comparison of knowledge‐based weaning (KBW) and physician‐driven weaning of mechanically ventilated patients in the coronary care unit. European Heart Journal 2012;33:714:P4188.
Liu 2013 {published and unpublished data}
-
- Liu L, Xu X, Yang Y, Huang Y, Liu S, Qiu H. Computer‐driven automated weaning reduces weaning duration in difficult‐to‐wean patients. Chinese Medical Journal 2013;126(10):1814‐8. - PubMed
Ma 2010 {published data only}
-
- Ma YJ, Yang XJ, Cao XY, Ma XG. Comparison of computer‐driven weaning and physician‐directed weaning from mechanical ventilation: a randomized prospective study. Zhonghua Jie He He Hu Xi Za Zhi 2010;33:174‐8. [PUBMED: 20450634] - PubMed
Papirov 2007 {unpublished data only}
-
- Papirov G. Computer driven management of weaning following prolonged mechanical ventilation: a pilot study. http://clinicaltrials.gov/show/NCT00502489. [NCT00502489]
Reardon 2011 {published data only}
-
- Reardon C. Clinical trial of a computer‐driven weaning system for patients requiring mechanical ventilation. ClinicalTrials.gov 2011. [NCT00606554]
Rose 2008 {published data only}
-
- Rose L, Presneill JJ, Johnston L, Cade JF. A randomized, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare/PS. Intensive Care Medicine 2008;34:1788‐95. - PubMed
Stahl 2009 {published and unpublished data}
-
- Stahl C, Dahmen G, Ziegler A, Muhl E. Comparison of automated protocol ‐based versus non‐protocol‐based physician‐directed weaning from mechanical ventilation: A controlled clinical trial. Intensivmed 2009;46:441‐6. [PUBMED: 21274740] - PubMed
Taniguchi 2009 {published data only}
Wong 2008 {unpublished data only}
-
- Wong J. Automated ventilator controlled weaning vs. daily spontaneous breathing trial in difficult to wean ICU patients. www.clinicaltrials.gov 2008; Vol. December. [NCT00813839]
Additional references
Argov 1979
-
- Argov Z, Mastaglia FL. Disorders of neuromuscular transmission caused by drugs. New England Journal of Medicine 1979;301:4090‐13. [MEDLINE: ] - PubMed
Banner 1997
-
- Banner MJ, Lampotang S, Blanch PB. Mechanical ventilation. In: Civetta JM editor(s). Critical Care. Philadelphia, PA: Lippincott‐Raven, 1997.
Barisione 1997
-
- Barisione G, Rovida S, Gazzaniga GM. Upper abdominal surgery: does a lung function test exist to predict early severe postoperative respiratory complications?. The European Respiratory Journal 1997;10:1301‐8. [MEDLINE: ] - PubMed
Bouadma 2005
-
- Bouadma L, Lellouche F, Cabello B, Taille S, Mancebo J, Dojat M, et al. Computer‐driven management of prolonged mechanical ventilation and weaning: A pilot study. Intensive Care Medicine 2005;10:1446‐50. [PUBMED: 16132889] - PubMed
Bowman 1993
-
- Bowman W. Physiology and pharmacology of neuromuscular transmission with special reference to the possible consequences of prolonged blockade. Intensive Care Medicine 1993;19:545‐53. [MEDLINE: ] - PubMed
Burns 2008
-
- Burns KEA, Lellouche F, Lessard M. Automating the weaning process with advanced closed loop systems. Intensive Care Medicine 2008;34:1757‐65. [MEDLINE: ] - PubMed
Burns 2010a
Burns 2010b
Dickerson 1994
Dureuil 1986
-
- Dureuil B, Viires N, Cantineau JP. Diaphragmatic contractility after upper abdominal surgery. Journal of Applied Physiology 1986;61:1775‐80. [MEDLINE: ] - PubMed
Egger 1997
Guyatt 2008
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011].The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Lee 1995
-
- Lee C. Intensive care unit neuromuscular syndrome?. Anesthesiology 1995;83:237‐40. [MEDLINE: ] - PubMed
Lefebvre 2001
-
- Lefebvre C, Clarke M. Identifying randomized trials. Egger M, Davey‐Smith G, Altman DG (editors). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ Books, 2001:69‐86.
Mircea 1982
-
- Mircea N, Constantinescu C, Jianu E. Risk of pulmonary complications in surgical patients. Resuscitation 1982;10:33‐41. [MEDLINE: ] - PubMed
Mitchell 1998
-
- Mitchell CK, Smoger SH, Pfeifer MP. Multivariate analysis of factors associated with pulmonary complications following elective general surgery. Archives of Surgery 1998;133:194‐8. [MEDLINE: ] - PubMed
Oxman 1992
-
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116:78‐84. [MEDLINE: ] - PubMed
Price 1999
-
- Price JA, Rizk NW. Postoperative ventilatory management. Chest 1999;115 Suppl:130‐7. [MEDLINE: ] - PubMed
RevMan 5.1 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robinson 2002
-
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. [MEDLINE: ] - PubMed
Strickland 1991
-
- Strickland JH, Hasson JH. A computer‐controlled ventilator weaning system. Chest 1991;100:1914564. [PUBMED: 1914564] - PubMed
Strickland 1993
-
- Strickland JH, Hasson JH. A computer‐controlled ventilator weaning system. Chest 1993;103:1220‐6. [MEDLINE: ] - PubMed
Viby‐Mogensen 1981
-
- Viby‐Mogensen J. Succinylcholine neuromuscular blockade in subjects homozygous for atypical plasma cholinesterase. Anesthesiology 1981;55:429‐34. [MEDLINE: ] - PubMed
Witt 1991
-
- Witt NJ, Zochodne DW, Bolton CF, Grand'Maison F, Wells G, Young GB, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest 1991;99:176‐84. [MEDLINE: ] - PubMed
Yusuf 1991
-
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266:93‐8. [MEDLINE: ] - PubMed
References to other published versions of this review
Burns 2010c
-
- Burns KEA, Lellouche F, Nisenbaum R, Lessard MR, Friedrich JO. SmartCare™ versus non‐automated mechanical ventilation strategies on discontinuation time for adults in the postoperative period. Cochrane Database of Systematic Reviews 2010, Issue 8. [CENTRAL: CD008639; DOI: 10.1002/14651858.CD008639] - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

