ADP ribosylation factor 6 (Arf6) acts through FilGAP protein to down-regulate Rac protein and regulates plasma membrane blebbing
- PMID: 24526684
- PMCID: PMC3975016
- DOI: 10.1074/jbc.M113.546051
ADP ribosylation factor 6 (Arf6) acts through FilGAP protein to down-regulate Rac protein and regulates plasma membrane blebbing
Abstract
The small GTP-binding protein Arf6 reorganizes the actin cytoskeleton through the regulation of Rac activity. We identified FilGAP, a Rac-specific Rho GTPase-activating protein that is recruited to plasma membranes by binding to activated Arf6. FilGAP binds to Arf6 through its pleckstrin homology domain. Activated Arf6 stimulated RacGAP activity of FilGAP, and knockdown of endogenous Arf6 by siRNA suppresses FilGAP-mediated bleb formation. Mutant FilGAP lacking phosphatidylinositol 3,4,5-trisphosphate (PIP3) binding (FilGAP R39C) binds to activated Arf6 and induces bleb formation. Moreover, bleb formation induced by wild-type FilGAP occurs in the presence of phosphatidylinositol 3-kinase inhibitors, suggesting a PIP3-independent interaction between FilGAP and Arf6. We propose that FilGAP may function as a mediator of the regulation of Rac by Arf6.
Keywords: Actin; Arf6; Cell Migration; Cell Polarization; Cytoskeleton; PI 3-kinase; Rac; Rho; Signal Transduction; Small GTPases.
Figures


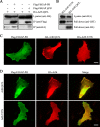
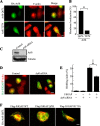
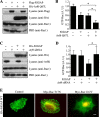
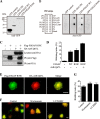
References
-
- Jaffe A. B., Hall A. (2005) Rho GTPases. Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 - PubMed
-
- Donaldson J. G., Honda A. (2005) Localization and function of Arf family GTPases. Biochem. Soc. Trans. 33, 639–642 - PubMed
-
- D'Souza-Schorey C., Chavrier P. (2006) ARF proteins. Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, 347–358 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

