Efficacy and safety of azithromycin 1.5% eye drops in paediatric population with purulent bacterial conjunctivitis
- PMID: 24526744
- PMCID: PMC4033170
- DOI: 10.1136/bjophthalmol-2013-303888
Efficacy and safety of azithromycin 1.5% eye drops in paediatric population with purulent bacterial conjunctivitis
Abstract
Objective: To determine the efficacy and safety of azithromycin 1.5% eye drops in a paediatric population with purulent bacterial conjunctivitis.
Patients and methods: This was a multicentre, international, randomised, investigator-masked study in 286 children with purulent discharge and bulbar conjunctival injection. Patients received either azithromycin 1.5% eye drops (twice daily for 3 days) or tobramycin 0.3% eye drops (every 2 h for 2 days, then four times daily for 5 days). Clinical signs were evaluated on day (D) 0, 3 and 7, and cultures on D0 and D7. The primary variable was the clinical cure (absence of bulbar conjunctival injection and discharge) on D3 in the worse eye for patients with positive cultures on D0.
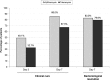
Results: 286 patients (mean age 3.2 years; range 1 day-17 years) were included; 203 had positive cultures on D0. Azithromycin was superior to tobramycin in clinical cure rate on D3 (47.1% vs 28.7%, p=0.013) and was non-inferior to tobramycin on D7 (89.2% vs 78.2%, respectively). Azithromycin treatment eradicated causative pathogens, including resistant species, with a similar resolution rate to tobramycin (89.8% vs 87.2%, respectively). These results were confirmed in a subgroup of patients younger than 24 months old.
Conclusions: Azithromycin 1.5% eye drops provided a more rapid clinical cure than tobramycin 0.3% eye drops in the treatment of purulent bacterial conjunctivitis in children, with a more convenient twice-a-day dosing regimen.
Keywords: Child Health (paediatrics); Conjunctiva; Infection.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Similar articles
-
Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis.SAGE Open Med. 2020 Sep 18;8:2050312120958846. doi: 10.1177/2050312120958846. eCollection 2020. SAGE Open Med. 2020. PMID: 32995001 Free PMC article. Review.
-
3-day treatment with azithromycin 1.5% eye drops versus 7-day treatment with tobramycin 0.3% for purulent bacterial conjunctivitis: multicentre, randomised and controlled trial in adults and children.Br J Ophthalmol. 2007 Apr;91(4):465-9. doi: 10.1136/bjo.2006.103556. Epub 2006 Oct 18. Br J Ophthalmol. 2007. PMID: 17050578 Free PMC article. Clinical Trial.
-
[Efficacy assessment of azithromycin 1.5% eye drops versus tobramycin 0.3% on clinical signs of purulent bacterial conjunctivitis].J Fr Ophtalmol. 2010 Apr;33(4):241-8. doi: 10.1016/j.jfo.2010.01.005. Epub 2010 Mar 10. J Fr Ophtalmol. 2010. PMID: 20223555 Clinical Trial. French.
-
Efficacy and safety of azithromycin 1.5% eye drops for purulent bacterial conjunctivitis in pediatric patients.Pediatr Infect Dis J. 2010 Mar;29(3):222-6. doi: 10.1097/INF.0b013e3181b99fa2. Pediatr Infect Dis J. 2010. PMID: 19935122 Clinical Trial.
-
Azithromycin 1.5% ophthalmic solution: in purulent bacterial or trachomatous conjunctivitis.Drugs. 2012 Feb 12;72(3):361-73. doi: 10.2165/11208580-000000000-00000. Drugs. 2012. PMID: 22316352 Review.
Cited by
-
Pediatric ocular nanomedicines: Challenges and opportunities.Chin Chem Lett. 2017 Sep;28(9):1817-1821. doi: 10.1016/j.cclet.2017.07.022. Epub 2017 Jul 26. Chin Chem Lett. 2017. PMID: 29147075 Free PMC article.
-
A 3-day regimen with azithromycin 1.5% eyedrops for the treatment of purulent bacterial conjunctivitis in children: efficacy on clinical signs and impact on the burden of illness.Clin Ophthalmol. 2015 Apr 20;9:725-32. doi: 10.2147/OPTH.S78747. eCollection 2015. Clin Ophthalmol. 2015. PMID: 25945033 Free PMC article.
-
An eye for azithromycin: review of the literature.Ther Adv Ophthalmol. 2018 Jul 30;10:2515841418783622. doi: 10.1177/2515841418783622. eCollection 2018 Jan-Dec. Ther Adv Ophthalmol. 2018. PMID: 30083656 Free PMC article. Review.
-
Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis.SAGE Open Med. 2020 Sep 18;8:2050312120958846. doi: 10.1177/2050312120958846. eCollection 2020. SAGE Open Med. 2020. PMID: 32995001 Free PMC article. Review.
-
Effectiveness and safety of azithromycin 1.5% eye drops for mass treatment of active trachoma in a highly endemic district in Cameroon.BMJ Open Ophthalmol. 2020 Nov 1;5(1):e000531. doi: 10.1136/bmjophth-2020-000531. eCollection 2020. BMJ Open Ophthalmol. 2020. PMID: 33195812 Free PMC article.
References
-
- McCormick M, Fleming D, Charlton J. Morbidity statistics from general practice: fourth national survey 1991–1992. London: HMSO, 1995
-
- Mah F. Bacterial conjunctivitis in pediatrics and primary care. Pediatr Clin North Am 2006;53:7–10 - PubMed
-
- Syed NA, Chandler JW. Bacterial conjunctivitis. In: Tabbara KF, Hyndiuk RA. eds. Infections of the eye. Vol. 8 2nd edn. Boston, USA: Little, Brown and Co., 1986:69–84
-
- Anonymous. Centers for disease control and prevention. Pneumococcal conjunctivitis at an elementary school-Maine, September 20-December 6, 2002. JAMA 2003;289:1097–8 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical