The Yin and Yang of Integrin Function in Re-Epithelialization During Wound Healing
- PMID: 24527329
- PMCID: PMC3840554
- DOI: 10.1089/wound.2011.0342
The Yin and Yang of Integrin Function in Re-Epithelialization During Wound Healing
Abstract
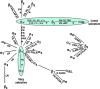
Background: Integrins are transmembrane proteins that are present in the plasma membrane of basal ketatinocytes and connect them to the underlying basement membrane and to the dermis. There are primarily two types of interactions between the epidermis and the dermis-via focal adhesion plaques and hemidesmosomes. It is critical that these interactions form properly to confer the skin strong mechanical properties. Integrins are also critical during wound healing, particularly in closure of the wound.
The problem: Margadant et al. (2009) address proper closure of cutaneous wounds. They developed a conditional knockout mouse for integrin α3 and showed that the absence of the α3 integrin resulted in faster migration of the keratinocytes during wound healing. However, its absence also led to inflammation, hair loss, basement membrane duplication, and loss of dermal epidermal interactions with blister formation. The latter has important consequences for the ability of the skin to withstand mechanical challenges.
Basic/clinical science advances: Models such as the conditional model developed by Margadant et al. (2009) will provide the opportunity for making major advances in understanding the complex function of integrins during healing.
Clinical care relevance: The model and the findings provide an opportunity to decipher mechanisms of disease and for potential development of treatments for human skin disorders and impaired healing, including chronic ulcers.
Conclusion: This work provides knowledge that leads to the understanding of delayed re-epithelialization during wound healing and dermal epidermal defects, blistering, and chronic skin diseases, hence providing the opportunity to understand the basic cellular and molecular mechanisms involved in these situations.
Figures


Similar articles
-
Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin.J Cell Sci. 2009 Jan 15;122(Pt 2):278-88. doi: 10.1242/jcs.029108. J Cell Sci. 2009. PMID: 19118220
-
Integrin-mediated regulation of epidermal wound functions.Cell Tissue Res. 2016 Sep;365(3):467-82. doi: 10.1007/s00441-016-2446-2. Epub 2016 Jun 28. Cell Tissue Res. 2016. PMID: 27351421 Free PMC article. Review.
-
A multiscale hybrid mathematical model of epidermal-dermal interactions during skin wound healing.Exp Dermatol. 2019 Apr;28(4):493-502. doi: 10.1111/exd.13909. Exp Dermatol. 2019. PMID: 30801791 Free PMC article.
-
Unique and redundant functions of integrins in the epidermis.FASEB J. 2010 Nov;24(11):4133-52. doi: 10.1096/fj.09-151449. Epub 2010 Jul 12. FASEB J. 2010. PMID: 20624931 Review.
-
Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing.J Invest Dermatol. 2009 Jan;129(1):217-28. doi: 10.1038/jid.2008.201. Epub 2008 Jul 17. J Invest Dermatol. 2009. PMID: 18633440 Free PMC article.
Cited by
-
Keratinocyte Function in Normal and Diabetic Wounds and Modulation by FOXO1.J Diabetes Res. 2020 Oct 28;2020:3714704. doi: 10.1155/2020/3714704. eCollection 2020. J Diabetes Res. 2020. PMID: 33195703 Free PMC article. Review.
-
The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds.Int J Mol Sci. 2023 Feb 21;24(5):4290. doi: 10.3390/ijms24054290. Int J Mol Sci. 2023. PMID: 36901720 Free PMC article. Review.
-
Cell-specific expression of the transcriptional regulator RHAMM provides a timing mechanism that controls appropriate wound re-epithelialization.J Biol Chem. 2020 Apr 17;295(16):5427-5448. doi: 10.1074/jbc.RA119.010002. Epub 2020 Mar 12. J Biol Chem. 2020. PMID: 32165498 Free PMC article.
-
Requirement for and polarized localization of integrin proteins during Drosophila wound closure.Mol Biol Cell. 2018 Sep 1;29(18):2137-2147. doi: 10.1091/mbc.E17-11-0635. Epub 2018 Jul 11. Mol Biol Cell. 2018. PMID: 29995573 Free PMC article.
References
-
- Margadant C. Charafeddine RA. Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133. - PubMed
-
- Dueck-Petreaca M. Martins-Green M. Cell-extracellular matrix interactions with implications for tissue engineering. In: Lanza R, editor; Langer R, editor; Vacanti J, editor. Principles of Tissue Engineering. 3rd. Academic Press; 2007. pp. 81–99.
-
- Ludwig RJ. Zillikens D. Pathogenesis of epidermolysis bullosa acquisita. Dermatol Clin. 2011;29:493. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

