Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing
- PMID: 24527344
- PMCID: PMC3857353
- DOI: 10.1089/wound.2012.0406
Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing
Abstract
Significance: This review highlights the critical role of transforming growth factor beta (TGF-β)1-3 within different phases of wound healing, in particular, late-stage wound healing. It is also very important to identify the TGF-β1-controlling factors involved in slowing down the healing process upon wound epithelialization.
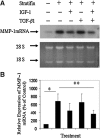
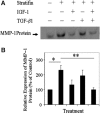
Recent advances: TGF-β1, as a growth factor, is a known proponent of dermal fibrosis. Several strategies to modulate or regulate TGF's actions have been thoroughly investigated in an effort to create successful therapies. This study reviews current discourse regarding the many roles of TGF-β1 in wound healing by modulating infiltrated immune cells and the extracellular matrix.
Critical issues: It is well established that TGF-β1 functions as a wound-healing promoting factor, and thereby if in excess it may lead to overhealing outcomes, such as hypertrophic scarring and keloid. Thus, the regulation of TGF-β1 in the later stages of the healing process remains as critical issue of which to better understand.
Future directions: One hypothesis is that cell communication is the key to regulate later stages of wound healing. To elucidate the role of keratinocyte/fibroblast cross talk in controlling the later stages of wound healing we need to: (1) identify those keratinocyte-released factors which would function as wound-healing stop signals, (2) evaluate the functionality of these factors in controlling the outcome of the healing process, and (3) formulate topical vehicles for these antifibrogenic factors to improve or even prevent the development of hypertrophic scarring and keloids as a result of deep trauma, burn injuries, and any type of surgical incision.
Figures

References
-
- Murphy JE. Robert C. Kupper TS. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol. 2000;114:602. - PubMed
-
- Richardson M. Acute wounds: an overview of the physiological healing process. Nurs Times. 2004;100:50. - PubMed
-
- Behm B. Babilas P. Landthaler M. Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26:812. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

