The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing
- PMID: 24527350
- PMCID: PMC3751266
- DOI: 10.1089/wound.2012.0398
The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing
Abstract
Significance: Postnatal vasculogenesis mediated via endothelial progenitor cells (EPCs) contributes to re-endothelialization and augments neovascularization after ischemia and tissue injury, providing a novel therapeutic application. However, controversy exists with respect to the origin, identification, and contributions of the EPCs to neovascularization, necessitating further study.
Recent advances: Bone marrow (BM) or circulating cells expressing cd133/vascular endothelial growth factor receptor 2 include those with endothelial progenitor capacity. Increasing evidence suggests that there are additional BM-derived (myeloid; mesenchymal cells) and non-BM-derived (peripheral and cord-blood; tissue-resident) cell populations which also give rise to endothelial cells (ECs) and contribute to re-endothelialization and growth factor release after ischemia and tissue injury. Currently, EPCs are being used as diagnostic markers for the assessment of cardiovascular and tumor risk/progression. Techniques aimed at enhancing ex vivo expansion and the therapeutic potential of these cells are being optimized.
Critical issues: Mobilization and EPC-mediated neovascularization are critically regulated. Stimulatory (growth factors, statins, and exercise) or inhibitory factors (obesity, diabetes, and other cardiovascular diseases) modulate EPC numbers and function. Recruitment and incorporation of EPCs require a coordinated sequence of signaling events, including adhesion, migration (by integrins), and chemoattraction. Finally, EPCs differentiate into ECs and/or secrete angiogenic growth factors. These cells are highly plastic, and depending on the microenvironment and presence of other cells, EPCs transdifferentiate and/or undergo cell fusion and become cells of a different lineage. Therefore, in vitro culture conditions should be optimized to mimic the in vivo milieu to fully characterize the biological function and contribution of EPCs to postnatal vasculogenesis.
Future directions: Advances in characterization of the EPC biology and enhancement of EPC functions are required. In addition, innovative tissue-engineered carrier matrices that permit embedding of EPCs and provide optimal conditions for EPC survival and endothelial outgrowth will further contribute to EPC-mediated therapeutic applications in wound healing and ischemia repair.
Figures

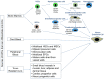




References
-
- Ko SH. Nauta A. Wong V. Glotzbach J. Gurtner GC. Longaker MT. The role of stem cells in cutaneous wound healing: what do we really know? Plast Reconstr Surg. 2011;127(Suppl 1):10S. - PubMed
-
- Asahara T. Masuda H. Takahashi T. Kalka C. Pastore C. Silver M. Kearne M. Magner M. Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221. - PubMed
-
- Real C. Caiado F. Dias S. Endothelial progenitors in vascular repair and angiogenesis: how many are needed and what to do? Cardiovasc Hematol Disord Drug Targets. 2008;8:185. - PubMed
-
- Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

