Neutrophils and Wound Repair: Positive Actions and Negative Reactions
- PMID: 24527354
- PMCID: PMC3763227
- DOI: 10.1089/wound.2012.0383
Neutrophils and Wound Repair: Positive Actions and Negative Reactions
Abstract
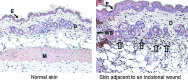
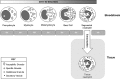
Significance: Neutrophils are one of the most abundant cells of the immune system and they are extremely active during the repair of cutaneous wounds. In general, the antimicrobial activity of neutrophils is effective and allows these cells to carry out their primary function of preventing wounds from becoming infected.
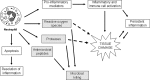
Recent advances: It is now known that in addition to sterilizing the wound, the weapons used by neutrophils to kill potential pathogens can also cause significant tissue damage to the host. This additional damage can lead to delayed healing and excessive scar formation.
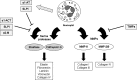
Critical issues: Much of the host damage caused by neutrophils results from the activity of proteases secreted by these cells. The clinical significance of this problem is highlighted by numerous studies showing that high levels of neutrophil-derived proteases are associated with chronic, non-healing wounds.
Future directions: Studies are currently being performed to evaluate new ways of counteracting protease activity in chronic wounds. Additional studies will have to be carried out to determine whether neutralizing neutrophil proteases can improve the healing of chronic wounds without sacrificing the ability of neutrophils to eliminate pathogens and risking infection.
Figures


References
-
- Theilgaard-Monch K. Knudsen S. Follin P. Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172:7684. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173. - PubMed
-
- Widgerow AD. Cellular resolution of inflammation—catabasis. Wound Repair Regen. 2012;20:2. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

