Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis
- PMID: 24527712
- PMCID: PMC4010687
- DOI: 10.1210/jc.2013-3186
Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis
Abstract
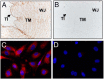
Context: The FSH receptor (FSHR) is traditionally thought to play a role in female reproductive physiology solely within the context of ovarian FSHR. However, FSHR is also expressed in endothelial cells of the placental vasculature and human umbilical cord vessels, suggesting additional facets of female reproduction regulated by extragonadal FSHR.
Objective: We sought to determine the functional role of FSHR on human umbilical cord endothelial cells (HUVECs), hypothesizing that activation of the FSHR would stimulate angiogenesis.
Design: The ability of FSH to stimulate several angiogenic processes in HUVECs was determined.
Setting: This was a laboratory-based study using commercially prepared HUVECs.
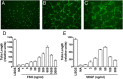
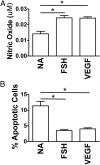
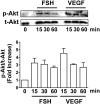
Results: Tube formation, wound healing, cell migration, cell proliferation, nitric oxide production, and cell survival were stimulated in response to FSH. Quantitative comparisons between HUVECs incubated with maximally stimulatory concentrations of FSH vs vascular endothelial growth factor (VEGF), a well-characterized angiogenic factor, revealed that FSH is as efficacious as VEGF in promoting angiogenic processes. FSH did not provoke increased secretion of VEGF by HUVECs, suggesting the direct stimulation of angiogenic processes by FSH in endothelial cells. In contrast to gonadal cells, the FSHR on HUVECs did not mediate an FSH-stimulated increase in cAMP. However, increased phosphorylation of AKT in response to FSH was observed, suggesting that FSH stimulation of HUVEC FSHR stimulates the PI3K/AKT signaling pathway.
Conclusions: Our studies reveal a novel role for FSHR in female reproductive physiology. Its ability to promote angiogenesis in placental endothelial cells suggests that the FSHR may have an influential role in pregnancy.
Figures


References
-
- Khankin EV, Royle C, Karumanchi SA. Placental vasculature in health and disease. Semin Thromb Hemost. 2010;36:309–320 - PubMed
-
- Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192 - PubMed
-
- Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621–1630 - PubMed
-
- Zhang M, Tao YX, Ryan GL, Feng X, Fanelli F, Segaloff DL. Intrinsic differences in the response of the human lutropin receptor versus the human follitropin receptor to activating mutations. J Biol Chem. 2007;282:25527–25539 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

