Approaches for classifying the indications for colonoscopy using detailed clinical data
- PMID: 24529031
- PMCID: PMC3927818
- DOI: 10.1186/1471-2407-14-95
Approaches for classifying the indications for colonoscopy using detailed clinical data
Abstract
Background: Accurate indication classification is critical for obtaining unbiased estimates of colonoscopy effectiveness and quality improvement efforts, but there is a dearth of published systematic classification approaches. The objective of this study was to evaluate the effects of data-source and adjudication on indication classification and on estimates of the effectiveness of screening colonoscopy on late-stage colorectal cancer diagnosis risk.
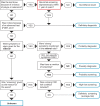
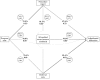
Methods: This was an observational study in members of four U.S. health plans. Eligible persons (n = 1039) were age 55-85 and had been enrolled for 5 years or longer in their health plans during 2006-2008. Patients were selected based on late-stage colorectal cancer diagnosis in a case-control design; each case patient was matched to 1-2 controls by study site, age, sex, and health plan enrollment duration. Reasons for colonoscopies received in the 10-year period before the reference date were collected from three medical records sources (progress notes; referral notes; procedure reports) and categorized using an algorithm, with committee adjudication of some tests. We evaluated indication classification concordance before and after adjudication and used logistic regressions with the Wald Chi-square test to compare estimates of the effects of screening colonoscopy on late-stage colorectal cancer diagnosis risk for each of our data sources to the adjudicated indication.
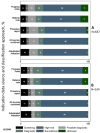
Results: Classification agreement between each data-source and adjudication was 78.8-94.0% (weighted kappa = 0.53-0.72); the highest agreement (weighted kappa = 0.86-0.88) was when information from all data sources was considered together. The choice of data-source influenced the association between screening colonoscopy and late-stage colorectal cancer diagnosis; estimates based on progress notes were closest to those based on the adjudicated indication (% difference in regression coefficients = 2.4%, p-value = 0.98), as compared to estimates from only referral notes (% difference in coefficients = 34.9%, p-value = 0.12) or procedure reports (% difference in coefficients = 27.4%, p-value = 0.23).
Conclusion: There was no single gold-standard source of information in medical records. The estimates of colonoscopy effectiveness from progress notes alone were the closest to estimates using adjudicated indications. Thus, the details in the medical records are necessary for accurate indication classification.
Figures

References
-
- Screening for colorectal cancer. U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;14(9):627–637. - PubMed
-
- Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. - DOI - PubMed
-
- Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. - DOI - PMC - PubMed
-
- Doubeni CA, Weinmann S, Adams K, Kamineni A, Buist DS, Ash AS, Rutter CM, Doria-Rose VP, Corley DA, Greenlee RT. et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case–control study. Ann Intern Med. 2013;158(5 Pt 1):312–320. - PMC - PubMed
-
- Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C. et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian randomized controlled trial–SCORE. J Natl Cancer Inst. 2011;103(17):1310–1322. doi: 10.1093/jnci/djr284. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

