Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation
- PMID: 24529478
- PMCID: PMC3991392
- DOI: 10.1016/j.cell.2014.01.023
Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation
Abstract
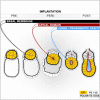
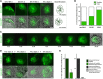
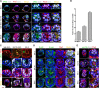
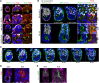
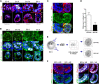
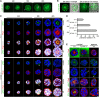
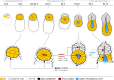
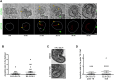
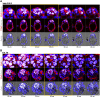
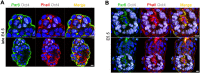
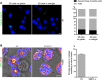
Transformation of pluripotent epiblast cells into a cup-shaped epithelium as the mouse blastocyst implants is a poorly understood and yet key developmental step. Studies of morphogenesis in embryoid bodies led to the current belief that it is programmed cell death that shapes the epiblast. However, by following embryos developing in vivo and in vitro, we demonstrate that not cell death but a previously unknown morphogenetic event transforms the amorphous epiblast into a rosette of polarized cells. This transformation requires basal membrane-stimulated integrin signaling that coordinates polarization of epiblast cells and their apical constriction, a prerequisite for lumenogenesis. We show that basal membrane function can be substituted in vitro by extracellular matrix (ECM) proteins and that ES cells can be induced to form similar polarized rosettes that initiate lumenogenesis. Together, these findings lead to a completely revised model for peri-implantation morphogenesis in which ECM triggers the self-organization of the embryo's stem cells.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Bedzhov I., Alotaibi H., Basilicata M.F., Ahlborn K., Liszewska E., Brabletz T., Stemmler M.P. Adhesion, but not a specific cadherin code, is indispensable for ES cell and induced pluripotency. Stem Cell Res. (Amst.) 2013;11:1250–1263. - PubMed
-
- Chazaud C., Yamanaka Y., Pawson T., Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell. 2006;10:615–624. - PubMed
-
- Chen F., Ma L., Parrini M.C., Mao X., Lopez M., Wu C., Marks P.W., Davidson L., Kwiatkowski D.J., Kirchhausen T. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr. Biol. 2000;10:758–765. - PubMed
Supplemental References
-
- Vestweber D., Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp. Cell Res. 1984;152:169–178. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

