Vitamin D metabolism, mechanism of action, and clinical applications
- PMID: 24529992
- PMCID: PMC3968073
- DOI: 10.1016/j.chembiol.2013.12.016
Vitamin D metabolism, mechanism of action, and clinical applications
Abstract
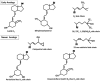
Vitamin D3 is made in the skin from 7-dehydrocholesterol under the influence of UV light. Vitamin D2 (ergocalciferol) is derived from the plant sterol ergosterol. Vitamin D is metabolized first to 25 hydroxyvitamin D (25OHD), then to the hormonal form 1,25-dihydroxyvitamin D (1,25(OH)2D). CYP2R1 is the most important 25-hydroxylase; CYP27B1 is the key 1-hydroxylase. Both 25OHD and 1,25(OH)2D are catabolized by CYP24A1. 1,25(OH)2D is the ligand for the vitamin D receptor (VDR), a transcription factor, binding to sites in the DNA called vitamin D response elements (VDREs). There are thousands of these binding sites regulating hundreds of genes in a cell-specific fashion. VDR-regulated transcription is dependent on comodulators, the profile of which is also cell specific. Analogs of 1,25(OH)2D are being developed to target specific diseases with minimal side effects. This review will examine these different aspects of vitamin D metabolism, mechanism of action, and clinical application.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 2008;4:404–412. - PubMed
-
- Adorini L, Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Fibbi B, Morelli A, Uskokovic M, Colli E, Maggi M. Inhibition of prostate growth and inflammation by the vitamin D receptor agonist BXL-628 (elocalcitol). J. Steroid Biochem. Mol. Biol. 2007;103:689–693. - PubMed
-
- Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006;57:234–240. - PubMed
-
- Armbrecht HJ, Hodam TL, Boltz MA, Partridge NC, Brown AJ, Kumar VB. Induction of the vitamin D 24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 is regulated by parathyroid hormone in UMR106 osteoblastic cells. Endocrinology. 1998;139:3375–3381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

