Estrogen biology: new insights into GPER function and clinical opportunities
- PMID: 24530924
- PMCID: PMC4040308
- DOI: 10.1016/j.mce.2014.02.002
Estrogen biology: new insights into GPER function and clinical opportunities
Abstract
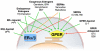
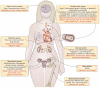
Estrogens play an important role in the regulation of normal physiology, aging and many disease states. Although the nuclear estrogen receptors have classically been described to function as ligand-activated transcription factors mediating genomic effects in hormonally regulated tissues, more recent studies reveal that estrogens also mediate rapid signaling events traditionally associated with G protein-coupled receptors. The G protein-coupled estrogen receptor GPER (formerly GPR30) has now become recognized as a major mediator of estrogen's rapid cellular effects throughout the body. With the discovery of selective synthetic ligands for GPER, both agonists and antagonists, as well as the use of GPER knockout mice, significant advances have been made in our understanding of GPER function at the cellular, tissue and organismal levels. In many instances, the protective/beneficial effects of estrogen are mimicked by selective GPER agonism and are absent or reduced in GPER knockout mice, suggesting an essential or at least parallel role for GPER in the actions of estrogen. In this review, we will discuss recent advances and our current understanding of the role of GPER and the activity of clinically used drugs, such as SERMs and SERDs, in physiology and disease. We will also highlight novel opportunities for clinical development towards GPER-targeted therapeutics, for molecular imaging, as well as for theranostic approaches and personalized medicine.
Keywords: 17β-Estradiol; Cardiac; Cardiovascular; GPR30; Immune; SERM.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz ER, Cappello AR, Dolce V, Abonante S, Pezzi V, Maggiolini M. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ. Health Perspect. 2008;116:1648–1655. - PMC - PubMed
-
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

