ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells
- PMID: 24531387
- PMCID: PMC11029007
- DOI: 10.1007/s00262-014-1525-z
ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells
Abstract
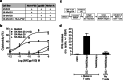
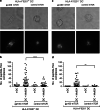
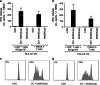
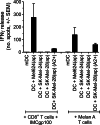
Antigen cross-presentation by dendritic cells (DCs) is thought to play a critical role in driving a polyclonal and durable T cell response against cancer. It follows, therefore, that the capacity of emerging immunotherapeutic agents to orchestrate tumour eradication may depend on their ability to induce antigen cross-presentation. ImmTACs [immune-mobilising monoclonal TCRs (T cell receptors) against cancer] are a new class of soluble bi-specific anti-cancer agents that combine pico-molar affinity TCR-based antigen recognition with T cell activation via a CD3-specific antibody fragment. ImmTACs specifically recognise human leucocyte antigen (HLA)-restricted tumour-associated antigens, presented by cancer cells, leading to T cell redirection and a potent anti-tumour response. Using an ImmTAC specific for a HLA-A*02-restricted peptide derived from the melanoma antigen gp100 (termed IMCgp100), we here observe that ImmTAC-driven melanoma-cell death leads to cross-presentation of melanoma antigens by DCs. These, in turn, can activate both melanoma-specific T cells and polyclonal T cells redirected by IMCgp100. Moreover, activation of melanoma-specific T cells by cross-presenting DCs is enhanced in the presence of IMCgp100; a feature that serves to increase the prospect of breaking tolerance in the tumour microenvironment. The mechanism of DC cross-presentation occurs via 'cross-dressing' which involves the rapid and direct capture by DCs of membrane fragments from dying tumour cells. DC cross-presentation of gp100-peptide-HLA complexes was visualised and quantified using a fluorescently labelled soluble TCR. These data demonstrate how ImmTACs engage with the innate and adaptive components of the immune system enhancing the prospect of mediating an effective and durable anti-tumour response in patients.
Conflict of interest statement
All authors are employees of Immunocore Ltd. Soluble monoclonal TCRs and ImmTACs, including IMCgp100, were produced by Immunocore Ltd.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

