Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity
- PMID: 24531506
- PMCID: PMC4043142
- DOI: 10.1016/j.mib.2013.12.005
Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity
Abstract
Salmonellae sense host cues to regulate properties important for bacterial survival and replication within host tissues. The PhoPQ two-component regulatory system senses phagosome acidification and cationic antimicrobial peptides (CAMP) to regulate the protein and lipid contents of the bacterial envelope that comprises an inner and outer membrane. PhoPQ-regulated lipid components of the outer membrane include lipopolysaccharides and glycerophospholipids. Envelope proteins regulated by PhoPQ, include: components of virulence associated secretion systems, the flagellar apparatus, membrane transport systems, and proteins that are likely structural components of the outer membrane. PhoPQ alteration of the bacterial surface results in increased bacterial resistance to CAMP and decreased detection by the innate immune system. This review details the molecular complexity of the bacterial cell envelope and highlights the outer membrane lipid bilayer as an environmentally regulated bacterial organelle.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

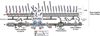
References
-
- Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6(1):53–66. - PubMed
-
-
Steeb B, Claudi B, Burton NA, Tienz P, Schmidt A, Farhan H, Maze A, Bumann D. Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog. 2013;9(4):e1003301. Proteomics, bacterial genetics, competitive infections, and computational analysis obtain a comprehensive overview of Salmonella metabolism and growth in a systemic model of infection. Lipid A and glycerophospholipids are synthesized from sugars, fatty acids, and amino acids. Therefore, studies like these will become increasingly important for under standing outer membrane remodeling mechanisms that occurs within specific host tissues.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

