Structure-based programming of lymph-node targeting in molecular vaccines
- PMID: 24531764
- PMCID: PMC4069155
- DOI: 10.1038/nature12978
Structure-based programming of lymph-node targeting in molecular vaccines
Abstract
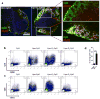
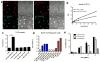
In cancer patients, visual identification of sentinel lymph nodes (LNs) is achieved by the injection of dyes that bind avidly to endogenous albumin, targeting these compounds to LNs, where they are efficiently filtered by resident phagocytes. Here we translate this 'albumin hitchhiking' approach to molecular vaccines, through the synthesis of amphiphiles (amph-vaccines) comprising an antigen or adjuvant cargo linked to a lipophilic albumin-binding tail by a solubility-promoting polar polymer chain. Administration of structurally optimized CpG-DNA/peptide amph-vaccines in mice resulted in marked increases in LN accumulation and decreased systemic dissemination relative to their parent compounds, leading to 30-fold increases in T-cell priming and enhanced anti-tumour efficacy while greatly reducing systemic toxicity. Amph-vaccines provide a simple, broadly applicable strategy to simultaneously increase the potency and safety of subunit vaccines.
Figures












References
-
- Salhab M, Patani N, Mokbel K. Sentinel lymph node micrometastasis in human breast cancer: an update. Surg Oncol. 2011;20:E195–E206. - PubMed
-
- Tsopelas C, Sutton R. Why certain dyes are useful for localizing the sentinel lymph node. J Nucl Med. 2002;43:1377–1382. - PubMed
-
- Johansen P, Mohanan D, Martínez-Gómez JM, Kündig TM, Gander B. Lympho-geographical concepts in vaccine delivery. J Controlled Release. 2010;148:56–62. - PubMed
-
- Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32:155–172. - PubMed

