Transdermal therapy for attention-deficit hyperactivity disorder with the methylphenidate patch (MTS)
- PMID: 24532028
- PMCID: PMC3933749
- DOI: 10.1007/s40263-014-0141-y
Transdermal therapy for attention-deficit hyperactivity disorder with the methylphenidate patch (MTS)
Abstract
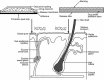
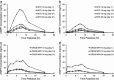
Transdermal technology is currently approved in the US for the administration of more than 20 medications. This current review describes the clinical research pertaining to the use of a methylphenidate patch in the treatment of attention-deficit hyperactivity disorder (ADHD) in children and adolescents. PubMed searches were conducted using the search term 'methylphenidate transdermal system', and were limited to clinical trials. No limits were set for dates of publication. A total of 21 citations were identified. Studies evaluating the safety and efficacy of the methylphenidate transdermal system (MTS) in children and adolescents were included in this review. Additional studies were identified from bibliographies and the 'Related Citations' section of PubMed searches. The MTS delivers a range of methylphenidate doses using a drug-in-adhesive matrix patch. According to current labeling, the patch should be applied to the hip once daily for a maximum of 9 h. Serum methylphenidate levels increase over wear time, with mean time to maximum concentration (t max) reached between 8 and 10 h for a 9-h wear time, and the elimination half-life for methylphenidate is 3-4 h after patch removal. In clinical trials, ADHD symptoms were measured using the ADHD Rating Scale, Version IV, and several parent-, teacher-, and patient-rated scales. Treatment effects show statistically significant differences from baseline symptom scores starting at the first evaluation, 2 h after the patch is applied, with significant benefit lasting up to 12 h with a 9-h wear time. Adverse events with the MTS are similar to those seen with other formulations of methylphenidate, with the exception of skin-related reactions at the site of application, which were generally mild to moderate in severity. The incidence of contact allergic dermatitis with MTS is <1%. Statistically significant improvements in health-related quality of life and medication satisfaction were also observed with the MTS compared with placebo, and after switching from oral extended-release (ER) methylphenidate. Transdermal drug delivery is an effective and safe means of administering methylphenidate for patients with ADHD.
Figures
References
-
- Neupro [package insert]. Smyrna (GA): UCB, Inc; 2012.
-
- Catapres-TTS [package insert]. Ridgefield (CT): Boehringer Ingelheim International GmBH; 2012.
-
- Daytrana [package insert]. Miami (FL): Noven Pharmaceuticals, Inc; 2010.
-
- Pierce D, Katic A, Buckwalter M, et al. Single- and multiple-dose pharmacokinetics of methylphenidate administered as methylphenidate transdermal system or osmotic-release oral system methylphenidate to children and adolescents with attention deficit hyperactivity disorder. J Clin Psychopharmacol. 2010;30(5):554–564. doi: 10.1097/JCP.0b013e3181f0c2f6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

