Safety and efficacy of switching to nilotinib 400 mg twice daily for patients with chronic myeloid leukemia in chronic phase with suboptimal response or failure on front-line imatinib or nilotinib 300 mg twice daily
- PMID: 24532039
- PMCID: PMC4077082
- DOI: 10.3324/haematol.2013.091272
Safety and efficacy of switching to nilotinib 400 mg twice daily for patients with chronic myeloid leukemia in chronic phase with suboptimal response or failure on front-line imatinib or nilotinib 300 mg twice daily
Abstract
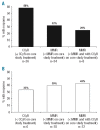
In a randomized, phase III trial of nilotinib versus imatinib in patients with newly diagnosed Philadelphia chromosome positive chronic myeloid leukemia in chronic phase, more patients had suboptimal response or treatment failure on front-line imatinib than on nilotinib. Patients with suboptimal response/treatment failure on imatinib 400 mg once or twice daily or nilotinib 300 mg twice daily could enter an extension study to receive nilotinib 400 mg twice daily. After a 19-month median follow up, the safety profile of nilotinib 400 mg twice daily in patients switching from imatinib (n=35) was consistent with previous reports, and few new adverse events occurred in patients escalating from nilotinib 300 mg twice daily (n=19). Of patients previously treated with imatinib or nilotinib 300 mg twice daily, respectively, 15 of 26 (58%) and 2 of 6 (33%) without complete cytogenetic response at extension study entry, and 11 of 34 (32%) and 7 of 18 (39%) without major molecular response at extension study entry, achieved these responses at any time on nilotinib 400 mg twice daily. Estimated 18-month rates of freedom from progression and overall survival after entering the extension study were lower for patients switched from imatinib (85% and 87%, respectively) versus nilotinib 300 mg twice daily (95% and 94%, respectively). Nilotinib dose escalation was generally well tolerated and improved responses in about one-third of patients with suboptimal response/treatment failure. Switch to nilotinib improved responses in some patients with suboptimal response/treatment failure on imatinib, but many did not achieve complete cytogenetic response (clinicaltrials.gov identifiers: 00718263, 00471497 - extension).
Trial registration: ClinicalTrials.gov NCT00471497 NCT00718263.
Copyright© Ferrata Storti Foundation.
Figures

Comment in
-
Extending the reach of nilotinib in chronic myeloid leukemia.Haematologica. 2014 Jul;99(7):1123-4. doi: 10.3324/haematol.2014.106195. Haematologica. 2014. PMID: 24986871 Free PMC article. No abstract available.
References
-
- Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant bcr-abl. Cancer Cell. 2005;7(2):129–41 - PubMed
-
- Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9 - PubMed
-
- Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–51 - PubMed
-
- Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–203 - PubMed
-
- Novartis Pharmaceuticals Corporation. Tasigna prescribing information. 2012

