Overstimulation of glutamate signals leads to hippocampal transcriptional plasticity in hamsters
- PMID: 24532154
- PMCID: PMC11488877
- DOI: 10.1007/s10571-014-0034-0
Overstimulation of glutamate signals leads to hippocampal transcriptional plasticity in hamsters
Abstract
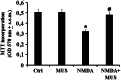
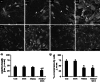
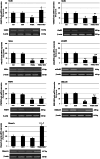
It's known that neurons in mammalian hibernators are more tolerant to hypoxia than those in non-hibernating species and as a consequence animals are capable of awakening from the arousal state without exhibiting cerebral damages. In addition, evidences have suggested that euthermic hamster neurons display protective adaptations against hypoxia, while those of rats are not capable, even though molecular mechanisms involved in similar neuroprotective strategies have not been yet fully studied. In the present work, overstimulation of glutamatergic receptors NMDA recognized as one of the major death-promoting element in hypoxia, accounted for altered network complexity consistent with a moderate reduction of hippocampal neuronal survival (p < 0.05) in hamsters. These alterations appeared to be featured concomitantly with altered glutamatergic signaling as indicated by significant down-regulation (p < 0.01) of NMDAergic (NR2A) and AMPAergic (GluR1, R2) receptor subtypes together with the metabotropic mGluR5 subtype. Diminished mRNA levels were also reported for NMDA receptor binding factors and namely PSD95 plus DREAM, which exert positive and negative regulatory properties, respectively, on receptor trafficking events. Conversely, involvement of glutamatergic signaling systems on neuronal excitotoxicity was strengthened by the co-activation of GABAAR-mediated effects as indicated by toxic morphological effects being notably reduced along with up-regulated GluR1, GluR2, mGluR5, DREAM, and Homer1c scaffold proteins when muscimol was added. Overall, these results point to a neuroprotective role of the GABAergic system against excitotoxicity episodes via DREAM-dependent inhibition of NMDA receptor and activation of AMPA receptor plus mGluR5, respectively, thus proposing them as novel therapeutic targets against cerebral ischemic damages in humans.
Conflict of interest statement
Dr. Anna Di Vito, Dr. Maria Mele, Dr. Antonella Piscioneri, Dr. Sabrina Morelli, Dr. Loredana De Bartolo, Dr. Tullio Barni, Dr. Rosa Maria Facciolo, Dr. Marcello Canonaco declares no competing financial interests.
Figures
Similar articles
-
Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review.
-
Metabotropic glutamate receptor 5 activation enhances tyrosine phosphorylation of the N-methyl-D-aspartate (NMDA) receptor and NMDA-induced cell death in hippocampal cultured neurons.Biol Pharm Bull. 2012;35(12):2224-9. doi: 10.1248/bpb.b12-00691. Biol Pharm Bull. 2012. PMID: 23207774
-
A light and electron microscopic study of glutamate receptors in the monkey subthalamic nucleus.J Neurocytol. 2000 Oct;29(10):743-54. doi: 10.1023/a:1010990404833. J Neurocytol. 2000. PMID: 11387548
-
Inorganic Polyphosphate Regulates AMPA and NMDA Receptors and Protects Against Glutamate Excitotoxicity via Activation of P2Y Receptors.J Neurosci. 2019 Jul 31;39(31):6038-6048. doi: 10.1523/JNEUROSCI.0314-19.2019. Epub 2019 May 30. J Neurosci. 2019. PMID: 31147524 Free PMC article.
-
The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity.Int J Mol Sci. 2017 Dec 27;19(1):69. doi: 10.3390/ijms19010069. Int J Mol Sci. 2017. PMID: 29280965 Free PMC article. Review.
Cited by
-
Extracellular Matrix Structure and Interaction with Immune Cells in Adult Astrocytic Tumors.Cell Mol Neurobiol. 2024 Jul 5;44(1):54. doi: 10.1007/s10571-024-01488-z. Cell Mol Neurobiol. 2024. PMID: 38969910 Free PMC article. Review.
-
Torpor enhances synaptic strength and restores memory performance in a mouse model of Alzheimer's disease.Sci Rep. 2021 Jul 29;11(1):15486. doi: 10.1038/s41598-021-94992-x. Sci Rep. 2021. PMID: 34326412 Free PMC article.
-
Dose-Dependent Effects of Zoledronic Acid on Human Periodontal Ligament Stem Cells: An In Vitro Pilot Study.Cell Transplant. 2020 Jan-Dec;29:963689720948497. doi: 10.1177/0963689720948497. Cell Transplant. 2020. PMID: 33086890 Free PMC article.
-
Application of the Co-culture Membrane System Pointed to a Protective Role of Catestatin on Hippocampal Plus Hypothalamic Neurons Exposed to Oxygen and Glucose Deprivation.Mol Neurobiol. 2017 Nov;54(9):7369-7381. doi: 10.1007/s12035-016-0240-5. Epub 2016 Nov 5. Mol Neurobiol. 2017. PMID: 27815840
-
In Vitro Long-Term Expansion and High Osteogenic Potential of Periodontal Ligament Stem Cells: More Than a Mirage.Cell Transplant. 2019 Jan;28(1):129-139. doi: 10.1177/0963689718807680. Epub 2018 Oct 28. Cell Transplant. 2019. PMID: 30369260 Free PMC article.
References
-
- Besancon E, Guo S, Lok J, Tymianski M, Lo EH (2008) Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29(5):268–275 - PubMed
-
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM (2007) Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res 1151:20–31 - PubMed
-
- Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40(5):e331–e339 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

