Cot/tpl2 participates in the activation of macrophages by adiponectin
- PMID: 24532642
- PMCID: PMC4021433
- DOI: 10.1189/jlb.0913486
Cot/tpl2 participates in the activation of macrophages by adiponectin
Abstract
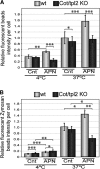
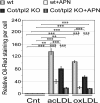
Whereas the main function of APN is to enhance insulin activity, it is also involved in modulating the macrophage phenotype. Here, we demonstrate that at physiological concentrations, APN activates Erk1/2 via the IKKβ-p105/NF-κΒ1-Cot/tpl2 intracellular signal transduction cassette in macrophages. In peritoneal macrophages stimulated with APN, Cot/tpl2 influences the ability to phagocytose beads. However, Cot/tpl2 did not modulate the known capacity of APN to decrease lipid content in peritoneal macrophages in response to treatment with oxLDL or acLDL. A microarray analysis of gene-expression profiles in BMDMs exposed to APN revealed that APN modulated the expression of ∼3300 genes; the most significantly affected biological functions were the inflammatory and the infectious disease responses. qRT-PCR analysis of WT and Cot/tpl2 KO macrophages stimulated with APN for 0, 3, and 18 h revealed that Cot/tpl2 participated in the up-regulation of APN target inflammatory mediators included in the cytokine-cytokine receptor interaction pathway (KEGG ID 4060). In accordance with these data, macrophages stimulated with APN increased secretion of cytokines and chemokines, including IL-1β, IL-1α, TNF-α, IL-10, IL-12, IL-6, and CCL2. Moreover, Cot/tpl2 also played an important role in the production of these inflammatory mediators upon stimulation of macrophages with APN. It has been reported that different types of signals that stimulate TLRs, IL-1R, TNFR, FcγR, and proteinase-activated receptor-1 activate Cot/tpl2. Here, we demonstrate that APN is a new signal that activates the IKKβ-p105/NF-κΒ1-Cot/tpl2-MKK1/2-Erk1/2 axis in macrophages. Furthermore, this signaling cassette modulates the biological functions triggered by APN in macrophages.
Keywords: M1 polarization; MAP kinases; fat-derived hormone; foam cells; mircroarray; phagocytosis.
© 2014 Society for Leukocyte Biology.
Figures







Similar articles
-
Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: Implications for NO synthase 2 expression.Eur J Immunol. 2011 Jun;41(6):1733-41. doi: 10.1002/eji.201041101. Epub 2011 May 24. Eur J Immunol. 2011. PMID: 21469113
-
Cot/Tpl2 regulates IL-23 p19 expression in LPS-stimulated macrophages through ERK activation.J Physiol Biochem. 2010 Mar;66(1):47-53. doi: 10.1007/s13105-010-0007-9. Epub 2010 Apr 20. J Physiol Biochem. 2010. PMID: 20405269
-
Sensitivity of TLR4- and -7-induced NF kappa B1 p105-TPL2-ERK pathway to TNF-receptor-associated-factor-6 revealed by RNAi in mouse macrophages.Mol Immunol. 2007 Jul;44(15):3715-23. doi: 10.1016/j.molimm.2007.04.002. Epub 2007 May 15. Mol Immunol. 2007. PMID: 17507094
-
TPL2 kinase action and control of inflammation.Pharmacol Res. 2018 Mar;129:188-193. doi: 10.1016/j.phrs.2017.11.031. Epub 2017 Nov 26. Pharmacol Res. 2018. PMID: 29183769 Review.
-
Tpl-2/Cot and COX-2 in breast cancer.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008 Jun;152(1):21-5. doi: 10.5507/bp.2008.003. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008. PMID: 18795070 Review.
Cited by
-
TPL-2 Regulates Macrophage Lipid Metabolism and M2 Differentiation to Control TH2-Mediated Immunopathology.PLoS Pathog. 2016 Aug 3;12(8):e1005783. doi: 10.1371/journal.ppat.1005783. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27487182 Free PMC article.
-
Obesity as an Inflammatory Agent Can Cause Cellular Changes in Human Milk due to the Actions of the Adipokines Leptin and Adiponectin.Cells. 2019 May 29;8(6):519. doi: 10.3390/cells8060519. Cells. 2019. PMID: 31146419 Free PMC article.
-
The Role of Invariant NKT in Autoimmune Liver Disease: Can Vitamin D Act as an Immunomodulator?Can J Gastroenterol Hepatol. 2018 Jun 26;2018:8197937. doi: 10.1155/2018/8197937. eCollection 2018. Can J Gastroenterol Hepatol. 2018. PMID: 30046564 Free PMC article. Review.
-
Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver.Curr Pathobiol Rep. 2015 Dec 1;3(4):243-252. doi: 10.1007/s40139-015-0094-y. Epub 2015 Sep 30. Curr Pathobiol Rep. 2015. PMID: 26858914 Free PMC article.
-
Map3k8 controls granulocyte colony-stimulating factor production and neutrophil precursor proliferation in lipopolysaccharide-induced emergency granulopoiesis.Sci Rep. 2017 Jul 10;7(1):5010. doi: 10.1038/s41598-017-04538-3. Sci Rep. 2017. PMID: 28694430 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

