Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results
- PMID: 24532785
- PMCID: PMC4215289
- DOI: 10.1136/jnnp-2013-306936
Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results
Abstract
Background: Clinical trials established the efficacy and safety of natalizumab. Data are needed over longer periods of time and in the clinical practice setting.
Objective: To evaluate long-term safety of natalizumab and its impact on annualised relapse rate and Expanded Disability Status Scale (EDSS) progression in patients with relapsing-remitting multiple sclerosis (RRMS).
Methods: The Tysabri (natalizumab) Observational Program (TOP) is an open-label, multinational, 10-year prospective study in clinical practice settings.
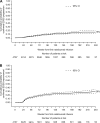
Results: In this 5-year interim analysis, 4821 patients were enrolled. Follow-up for at least 4 years from natalizumab commencement in 468 patients and at least 2 years in 2496 patients revealed no new safety signals. There were 18 cases of progressive multifocal leucoencephalopathy reported, following 11-44 natalizumab infusions. Mean annualised relapse rate decreased from 1.99 in the 12 months prior to baseline to 0.31 on natalizumab therapy (p<0.0001), remaining low at 5 years. Lower annualised relapse rates were observed in patients who used natalizumab as first MS therapy, in patients with lower baseline EDSS scores, and in patients with lower prenatalizumab relapse rates. Mean EDSS scores remained unchanged up to 5 years.
Conclusions: Interim TOP data confirm natalizumab's overall safety profile and the low relapse rate and stabilised disability levels in natalizumab-treated patients with RRMS in clinical practice.
Trial registration number: NCT00493298.
Keywords: Multiple Sclerosis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


Comment in
-
Natalizumab in clinical practice: managing the risks, enjoying the benefits.J Neurol Neurosurg Psychiatry. 2014 Nov;85(11):1181. doi: 10.1136/jnnp-2013-307355. Epub 2014 Mar 21. J Neurol Neurosurg Psychiatry. 2014. PMID: 24659794 No abstract available.
References
-
- Rudick RA, Sandrock A. Natalizumab: α4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother 2004;4:571–80. - PubMed
-
- Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. - PubMed
-
- Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 2009;256:405–15. - PubMed
-
- Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 2005;64:1144–51. - PubMed
-
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
