Effect of armodafinil on cortical activity and working memory in patients with residual excessive sleepiness associated with CPAP-Treated OSA: a multicenter fMRI study
- PMID: 24532997
- PMCID: PMC3899316
- DOI: 10.5664/jcsm.3440
Effect of armodafinil on cortical activity and working memory in patients with residual excessive sleepiness associated with CPAP-Treated OSA: a multicenter fMRI study
Abstract
Study objective: To assess the effect of armodafinil on task-related prefrontal cortex activation using functional magnetic resonance imaging (fMRI) in patients with obstructive sleep apnea (OSA) and excessive sleepiness despite continuous positive airway pressure (CPAP) therapy.
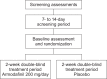
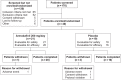
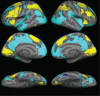
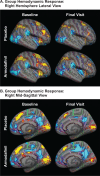
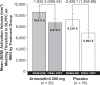
Methods: This 2-week, multicenter, prospective, randomized, double-blind, placebo-controlled, parallel-group study was conducted at five neuroimaging sites and four collaborating clinical study centers in the United States. Patients were 40 right-handed or ambidextrous men and women aged between 18 and 60 years, with OSA and persistent sleepiness, as determined by multiple sleep latency and Epworth Sleepiness Scale scores, despite effective, stable use of CPAP. Treatment was randomized (1:1) to once-daily armodafinil 200 mg or placebo. The primary efficacy outcome was a change from baseline at week 2 in the volume of activation meeting the predefined threshold in the dorsolateral prefrontal cortex during a 2-back working memory task. The key secondary measure was the change in task response latency.
Results: No significant differences were observed between treatment groups in the primary or key secondary outcomes. Armodafinil was generally well tolerated. The most common adverse events (occurring in more than one patient [5%]) were headache (19%), nasopharyngitis (14%), and diarrhea (10%).
Conclusions: Armodafinil did not improve fMRI-measured functional brain activation in CPAP-treated patients with OSA and excessive sleepiness.
Study registration: Double-Blind, Placebo-Controlled, Functional Neuroimaging Study of Armodafinil (200 mg/Day) on Prefrontal Cortical Activation in Patients With Residual Excessive Sleepiness Associated With Obstructive Sleep Apnea/Hypopnea.
Trial registration: ClinicalTrials.gov NCT00711516.
Keywords: 2-back task; Armodafinil; cortical activation; excessive sleepiness; functional magnetic resonance imaging; neuroimaging; obstructive sleep apnea.
Figures
References
-
- Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. - PubMed
-
- Malhotra A. Obstructive sleep apnoea. Lancet. 2002;360:237–45. - PubMed
-
- Pankow W, Nabe B, Lies A, Becker H, Köhler U, Kohl FV, et al. Influence of sleep apnea on 24-hour blood pressure. Chest. 1997;112:1253–8. - PubMed
-
- McNicholas WT, Bonsignore MR. Management Committee of EU Cost Action B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. - PubMed
-
- Sahlman J, Miettinen K, Peuhkurinen K, et al. Kuopio Sleep Apnoea Group. The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnea. J Sleep Res. 2010;19:341–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

