Safety and efficacy of gliclazide as treatment for type 2 diabetes: a systematic review and meta-analysis of randomized trials
- PMID: 24533045
- PMCID: PMC3922704
- DOI: 10.1371/journal.pone.0082880
Safety and efficacy of gliclazide as treatment for type 2 diabetes: a systematic review and meta-analysis of randomized trials
Abstract
Objective and design: Gliclazide has been associated with a low risk of hypoglycemic episodes and beneficial long-term cardiovascular safety in observational cohorts. The aim of this study was to assess in a systematic review and meta-analysis of randomized controlled trials the safety and efficacy of gliclazide compared to other oral glucose-lowering agents (PROSPERO2013:CRD42013004156).
Data sources: Medline, EMBASE, Clinicaltrials.gov, Trialregister.nl, Clinicaltrialsregister.eu and the Cochrane database.
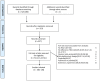
Selection: Included were randomized studies of at least 12 weeks duration with the following outcomes: HbA1c change, incidence of severe hypoglycemia, weight change, cardiovascular events and/or mortality when comparing gliclazide with other oral blood glucose lowering drugs. Bias was assessed with the Cochrane risk of bias tool. The inverse variance random effects model was used.
Results: Nineteen trials were included; 3,083 patients treated with gliclazide and 3,155 patients treated with other oral blood glucose lowering drugs. There was a considerable amount of heterogeneity between and bias in studies. Compared to other glucose lowering agents except metformin, gliclazide was slightly more effective (-0.13% (95%CI: -0.25, -0.02, I(2) 55%)). One out of 2,387 gliclazide users experienced a severe hypoglycemic event, whilst also using insulin. There were 25 confirmed non-severe hypoglycemic events (2.2%) in 1,152 gliclazide users and 22 events (1.8%) in 1,163 patients in the comparator group (risk ratio 1.09 (95% CI: 0.20, 5.78, I² 77%)). Few studies reported differences in weight and none were designed to evaluate cardiovascular outcomes.
Conclusions: The methodological quality of randomized trials comparing gliclazide to other oral glucose lowering agents was poor and effect estimates on weight were limited by publication bias. The number of severe hypoglycemic episodes was extremely low, and gliclazide appears at least equally effective compared to other glucose lowering agents. None of the trials were designed for evaluating cardiovascular outcomes, which warrants attention in future randomized trials.
Conflict of interest statement
Figures

References
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: 1364–1379. - PMC - PubMed
-
- (2008). Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London. - PubMed
-
- Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, et al. (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 32: 1900–1908. - PubMed
-
- Sadikot SM, Mogensen CE (2008) Risk of coronary artery disease associated with initial sulphonylurea treatment of patients with type 2 diabetes: a matched case-control study. Diabetes Res Clin Pract 82: 391–395. - PubMed
-
- Khalangot M, Tronko M, Kravchenko V, Kovtun V (2009) Glibenclamide-related excess in total and cardiovascular mortality risks: data from large Ukrainian observational cohort study. Diabetes Res Clin Pract 86: 247–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

