Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations
- PMID: 24533047
- PMCID: PMC3922700
- DOI: 10.1371/journal.pone.0085245
Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations
Abstract
Background: Several EGFR-tyrosine kinase inhibitors (EGFR-TKIs) including erlotinib, gefitinib, afatinib and icotinib are currently available as treatment for patients with advanced non-small-cell lung cancer (NSCLC) who harbor EGFR mutations. However, no head to head trials between these TKIs in mutated populations have been reported, which provides room for indirect and integrated comparisons.
Methods: We searched electronic databases for eligible literatures. Pooled data on objective response rate (ORR), progression free survival (PFS), overall survival (OS) were calculated. Appropriate networks for different outcomes were established to incorporate all evidences. Multiple-treatments comparisons (MTCs) based on Bayesian network integrated the efficacy and specific toxicities of all included treatments.
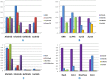
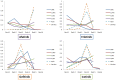
Results: Twelve phase III RCTs that investigated EGFR-TKIs involving 1821 participants with EGFR mutation were included. For mutant patients, the weighted pooled ORR and 1-year PFS of EGFR-TKIs were significant superior to that of standard chemotherapy (ORR: 66.6% vs. 30.9%, OR 5.46, 95%CI 3.59 to 8.30, P<0.00001; 1-year PFS: 42.9% vs. 9.7%, OR 7.83, 95%CI 4.50 to 13.61; P<0.00001) through direct meta-analysis. In the network meta-analyses, no statistically significant differences in efficacy were found between these four TKIs with respect to all outcome measures. Trend analyses of rank probabilities revealed that the cumulative probabilities of being the most efficacious treatments were (ORR, 1-year PFS, 1-year OS, 2-year OS): erlotinib (51%, 38%, 14%, 19%), gefitinib (1%, 6%, 5%, 16%), afatinib (29%, 27%, 30%, 27%) and icotinib (19%, 29%, NA, NA), respectively. However, afatinib and erlotinib showed significant severer rash and diarrhea compared with gefitinib and icotinib.
Conclusions: The current study indicated that erlotinib, gefitinib, afatinib and icotinib shared equivalent efficacy but presented different efficacy-toxicity pattern for EGFR-mutated patients. Erlotinib and afatinib revealed potentially better efficacy but significant higher toxicities compared with gefitinib and icotinib.
Conflict of interest statement
Figures



References
-
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300. - PubMed
-
- Ramalingam S, Belani C (2008) Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 13 Suppl. 1: 5–13. - PubMed
-
- Cataldo VD, Gibbons DL, Pérez-Soler R, Quintás-Cardama A (2011) Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med Mar 10: 947–55. - PubMed
-
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, et al. (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 21: 2866–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

