Clobazam and its active metabolite N-desmethylclobazam display significantly greater affinities for α₂- versus α₁-GABA(A)-receptor complexes
- PMID: 24533090
- PMCID: PMC3922815
- DOI: 10.1371/journal.pone.0088456
Clobazam and its active metabolite N-desmethylclobazam display significantly greater affinities for α₂- versus α₁-GABA(A)-receptor complexes
Abstract
Clobazam (CLB), a 1,5-benzodiazepine (BZD), was FDA-approved in October 2011 for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years and older. BZDs exert various CNS effects through allosteric modulation of GABAA receptors. The structurally distinct, 1,4-BZD clonazepam (CLN) is also approved to treat LGS. The precise mechanisms of action and clinical efficacy of both are unknown. Data show that the GABAA α₁-subunit-selective compound zolpidem [ZOL] exhibits hypnotic/sedative effects. Conversely, data from knock-in mice carrying BZD binding site mutations suggest that the α₂ subunit mediates anticonvulsant effects, without sedative actions. Hence, the specific pattern of interactions across the GABAA receptor complexes of BZDs might be reflected in their clinical efficacies and adverse effect profiles. In this study, GABAA-receptor binding affinities of CLB, N-desmethylclobazam (N-CLB, the major metabolite of CLB), CLN, and ZOL were characterized with native receptors from rat-brain homogenates and on cloned receptors from HEK293 cells transfected with combinations of α (α₁, α₂, α₃, or α₅), β₂, and γ₂ subtypes. Our results demonstrate that CLB and N-CLB have significantly greater binding affinities for α₂- vs. α₁-receptor complexes, a difference not observed for CLN, for which no distinction between α₂ and α₁ receptors was observed. Our experiments with ZOL confirmed the high preference for α₁ receptors. These results provide potential clues to a new understanding of the pharmacologic modes of action of CLB and N-CLB.
Conflict of interest statement
Figures
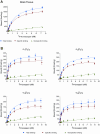
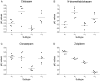
References
-
- Berezhnoy D, Gravielle MC, Farb DH (2007) Pharmacology of the GABAA Receptor, in Handbook of Contemporary Neuropharmacology, John Wiley & Sons, Inc.
-
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, et al. (1999) Benzodiazepine actions mediated by specific GABAA receptor subtypes. Nature 401: 796–800. - PubMed
-
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha1 subtype. Nat Neurosci 3: 587–592. - PubMed
-
- Fradley RL, Guscott MR, Bull S, Hallett DJ, Goodacre SC, et al. (2007) Differential contribution of GABAA receptor subtypes to the anticonvulsant efficacy of benzodiazepine site ligands. J Psychopharmacol 21: 384–391. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

