Candidate markers associated with the probability of future pulmonary exacerbations in cystic fibrosis patients
- PMID: 24533110
- PMCID: PMC3922941
- DOI: 10.1371/journal.pone.0088567
Candidate markers associated with the probability of future pulmonary exacerbations in cystic fibrosis patients
Abstract
Introduction: Pulmonary exacerbations (PEs) cause significant morbidity and can severely impact disease progression in cystic fibrosis (CF) lung disease, especially in patients who suffer from recurrent PEs. The assessments able to predict a future PE or a recurrent PE are limited. We hypothesized that combining clinical, molecular and patient reported data could identify patients who are at risk of PE.
Methods: We prospectively followed a cohort of 53 adult CF patients for 24 months. Baseline values for spirometry, clinical status using the Matouk Disease Score, quality of life (QOL), inflammatory markers (C-reactive protein (CRP), interleukins (IL)-1β, -6, -8, -10, macrophage inflammatory protein (MIP)-1β, tumor necrosis factor (TNF) and vascular endothelial growth factor (VEGF)), polyunsaturated fatty acids and lipid peroxidation in blood plasma were collected for all patients during periods of stable disease, and patients were monitored for PE requiring PO/IV antibiotic treatment. Additionally, we closely followed 13 patients during PEs collecting longitudinal data on changes in markers from baseline values. We assessed whether any markers were predictors of future PE at baseline and after antibiotic treatment.
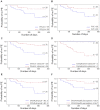
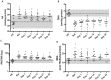
Results: Out of 53 patients, 37 experienced PEs during our study period. At baseline, we found that low lung function, clinical scoring and QOL values were associated with increased risk of PE events. PEs were associated with increased inflammatory markers at Day 1, and these biomarkers improved with treatment. The imbalance in arachidonic acid and docosahexaenoic acid levels improved with treatment which coincided with reductions in lipid peroxidation. High levels of inflammatory markers CRP and IL-8 were associated with an early re-exacerbation.
Conclusion: Our results demonstrate that worse clinical and QOL assessments during stable disease are potential markers associated with a higher risk of future PEs, while higher levels of inflammatory markers at the end of antibiotic treatment may be associated with early re-exacerbation.
Conflict of interest statement
Figures


References
-
- Parkins MD, Rendall JC, Elborn JS (2012) Incidence and risk factors for pulmonary exacerbation treatment failures in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. Chest 141: 485–493. - PubMed
-
- Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, et al. (2012) Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J 40: 61–66. - PubMed
-
- de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, et al. (2011) Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 66: 680–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

