Aggregate-depleted brain fails to induce Aβ deposition in a mouse model of Alzheimer's disease
- PMID: 24533166
- PMCID: PMC3923072
- DOI: 10.1371/journal.pone.0089014
Aggregate-depleted brain fails to induce Aβ deposition in a mouse model of Alzheimer's disease
Abstract
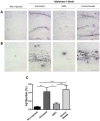
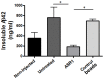
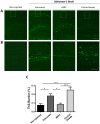
Recent studies in animal models of Alzheimer's disease (AD) show that amyloid-beta (Aβ) misfolding can be transmissible; however, the mechanisms by which this process occurs have not been fully explored. The goal of this study was to analyze whether depletion of aggregates from an AD brain suppresses its in vivo "seeding" capability. Removal of aggregates was performed by using the Aggregate Specific Reagent 1 (ASR1) compound which has been previously described to specifically bind misfolded species. Our results show that pre-treatment with ASR1-coupled magnetic beads reduces the in vivo misfolding inducing capability of an AD brain extract. These findings shed light respect to the active principle responsible for the prion-like spreading of Alzheimer's amyloid pathology and open the possibility of using seeds-capturing reagents as a promising target for AD treatment.
Conflict of interest statement
Figures

References
-
- Gomez-Isla T, Spires T, de CA, Hyman BT (2008) Neuropathology of Alzheimer's disease. Handb Clin Neurol 89: 233–243. - PubMed
-
- Glabe CG (2006) Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging 27: 570–575. - PubMed
-
- Estus S, Borchelt D, Kindy MS, Vassar R (2002) Abeta deposition is essential to AD neuropathology. J Alzheimers Dis 4: 133–138. - PubMed
-
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

