The proteasome of malaria parasites: A multi-stage drug target for chemotherapeutic intervention?
- PMID: 24533266
- PMCID: PMC3862440
- DOI: 10.1016/j.ijpddr.2011.12.001
The proteasome of malaria parasites: A multi-stage drug target for chemotherapeutic intervention?
Abstract
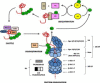
The ubiquitin/proteasome system serves as a regulated protein degradation pathway in eukaryotes, and is involved in many cellular processes featuring high protein turnover rates, such as cell cycle control, stress response and signal transduction. In malaria parasites, protein quality control is potentially important because of the high replication rate and the rapid transformations of the parasite during life cycle progression. The proteasome is the core of the degradation pathway, and is a major proteolytic complex responsible for the degradation and recycling of non-functional ubiquitinated proteins. Annotation of the genome for Plasmodium falciparum, the causative agent of malaria tropica, revealed proteins with similarity to human 26S proteasome subunits. In addition, a bacterial ClpQ/hslV threonine peptidase-like protein was identified. In recent years several independent studies indicated an essential function of the parasite proteasome for the liver, blood and transmission stages. In this review, we compile evidence for protein recycling in Plasmodium parasites and discuss the role of the 26S proteasome as a prospective multi-stage target for antimalarial drug discovery programs.
Keywords: Inhibitor; Plasmodium falciparum; Proteasome; Ubiquitin.
Figures


References
-
- Aurrecoechea C., Brestelli J., Brunk B.P., Dommer J., Fischer S., Gajria B., Gao X., Gingle A., Grant G., Harb O.S., Heiges M., Innamorato F., Iodice J., Kissinger J.C., Kraemer E., Li W., Miller J.A., Nayak V., Pennington C., Pinney D.F., Roos D.S., Ross C., Stoeckert C.J., Jr, Treatman C., Wang H. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–543. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

