Moxidectin and the avermectins: Consanguinity but not identity
- PMID: 24533275
- PMCID: PMC3862425
- DOI: 10.1016/j.ijpddr.2012.04.001
Moxidectin and the avermectins: Consanguinity but not identity
Abstract
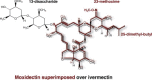
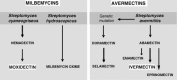
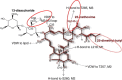
The avermectins and milbemycins contain a common macrocyclic lactone (ML) ring, but are fermentation products of different organisms. The principal structural difference is that avermectins have sugar groups at C13 of the macrocyclic ring, whereas the milbemycins are protonated at C13. Moxidectin (MOX), belonging to the milbemycin family, has other differences, including a methoxime at C23. The avermectins and MOX have broad-spectrum activity against nematodes and arthropods. They have similar but not identical, spectral ranges of activity and some avermectins and MOX have diverse formulations for great user flexibility. The longer half-life of MOX and its safety profile, allow MOX to be used in long-acting formulations. Some important differences between MOX and avermectins in interaction with various invertebrate ligand-gated ion channels are known and could be the basis of different efficacy and safety profiles. Modelling of IVM interaction with glutamate-gated ion channels suggest different interactions will occur with MOX. Similarly, profound differences between MOX and the avermectins are seen in interactions with ABC transporters in mammals and nematodes. These differences are important for pharmacokinetics, toxicity in animals with defective transporter expression, and probable mechanisms of resistance. Resistance to the avermectins has become widespread in parasites of some hosts and MOX resistance also exists and is increasing. There is some degree of cross-resistance between the avermectins and MOX, but avermectin resistance and MOX resistance are not identical. In many cases when resistance to avermectins is noticed, MOX produces a higher efficacy and quite often is fully effective at recommended dose rates. These similarities and differences should be appreciated for optimal decisions about parasite control, delaying, managing or reversing resistances, and also for appropriate anthelmintic combination.
Keywords: ATP binding cassette transporter; Avermectin; Ivermectin; Ligand-gated ion channels; Moxidectin; Nematode; Toxicity.
Figures
References
-
- Al-Azzam S.I., Fleckenstein L., Cheng K.J., Dzimianski M.T., McCall J.W. Comparison of the pharmacokinetics of moxidectin and ivermectin after oral administration to beagle dogs. Biopharm. Drug Dispos. 2007;28:431–438. - PubMed
-
- Almeida F.A., Garcia K.C., Torgerson P.R., Amarante A.F. Multiple resistance to anthelmintics by Haemonchus contortus and Trichostrongylus colubriformis in sheep in Brazil. Parasitol. Int. 2010;59:622–625. - PubMed
-
- Alvinerie M., Sutra J.F., Galtier P. Ivermectin in goat plasma and milk after subcutaneous injection. Vet. Res. 1993;24:417–421. - PubMed
-
- Alvinerie M., Lacoste E., Sutra J.F., Chartier C. Some pharmacokinetic parameters of eprinomectin in goats following pour-on administration. Vet. Res. Commun. 1999;23:449–455. - PubMed
-
- Alvinerie M., Sutra J.F., Galtier P., Lifschitz A., Virkel G., Sallovitz J., Lanusse C. Persistence of ivermectin in plasma and faeces following administration of a sustained-release bolus to cattle. Res. Vet. Sci. 1999;66:57–61. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

