Effects of the green tea catechin (-)-epigallocatechin gallate on Trypanosoma brucei
- PMID: 24533284
- PMCID: PMC3862400
- DOI: 10.1016/j.ijpddr.2012.09.001
Effects of the green tea catechin (-)-epigallocatechin gallate on Trypanosoma brucei
Abstract
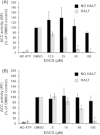
The current pharmacopeia to treat the lethal human and animal diseases caused by the protozoan parasite Trypanosoma brucei remains limited. The parasite's ability to undergo antigenic variation represents a considerable barrier to vaccine development, making the identification of new drug targets extremely important. Recent studies have demonstrated that fatty acid synthesis is important for growth and virulence of Trypanosoma brucei brucei, suggesting this pathway may have therapeutic potential. The first committed step of fatty acid synthesis is catalyzed by acetyl-CoA carboxylase (ACC), which is a known target of (-)-epigallocatechin-3-gallate (EGCG), an active polyphenol compound found in green tea. EGCG exerts its effects on ACC through activation of AMP-dependent protein kinase, which phosphorylates and inhibits ACC. We found that EGCG inhibited TbACC activity with an EC50 of 37 μM and 55 μM for bloodstream form and procyclic form lysates, respectively. Treatment with 100 μM EGCG induced a 4.7- and 1.7- fold increase in TbACC phosphorylation in bloodstream form and procyclic lysates. EGCG also inhibited the growth of bloodstream and procyclic parasites in culture, with a 48 h EC50 of 33 μM and 27 μM, respectively, which is greater than the EGCG plasma levels typically achievable in humans through oral dosing. Daily intraperitoneal administration of EGCG did not reduce the virulence of an acute mouse model of T. b. brucei infection. These data suggest a reduced potential for EGCG to treat T. brucei infections, but suggest that EGCG may prove to be useful as a tool to probe ACC regulation.
Keywords: ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; Acetyl-CoA carboxylase; BF, bloodstream form; DMSO, dimethyl sulfoxide; EGCG, (−)-epigallocatechin gallate; Epigallocatechin gallate; PF, procyclic form; Phosphorylation; RNAi, RNA interference; SA-HRP, streptavidin conjugated to horseradish peroxidase; Trypanosoma brucei.
Figures



References
-
- Bacchi C.J., Barker R.H., Jr., Rodriguez A., Hirth B., Rattendi D., Yarlett N., Hendrick C.L., Sybertz E. Trypanocidal activity of 8-methyl-5′-{[(Z)-4-aminobut-2-enyl]-(methylamino)}adenosine (Genz-644131), an adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 2009;53:3269–3272. - PMC - PubMed
-
- Barber M.C., Price N.T., Travers M.T. Structure and regulation of acetyl-CoA carboxylase genes of metazoa. Biochim. Biophys. Acta. 2005;1733:1–28. - PubMed
-
- Brownsey R.W., Boone A.N., Elliott J.E., Kulpa J.E., Lee W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006;34:223–227. - PubMed
-
- Brun R., Shonenberger M. Cultivation and in vitro cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 1979;36:289–292. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

