Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: a potential challenge for malaria vector control in Uganda
- PMID: 24533773
- PMCID: PMC3937429
- DOI: 10.1186/1756-3305-7-71
Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: a potential challenge for malaria vector control in Uganda
Abstract
Background: Although the An. funestus group conceals one of the major malaria vectors in Africa, little is known about the dynamics of members of this group across the continent. Here, we investigated the species composition, infection rate and susceptibility to insecticides of this species group in Uganda.
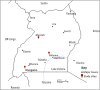
Methods: Indoor resting blood-fed Anopheles adult female mosquitoes were collected from 3 districts in Uganda. Mosquitoes morphologically belonging to the An. funestus group were identified to species by PCR. The sporozoite infection rates were determined by TaqMan and a nested PCR. Susceptibility to major insecticides was assessed using WHO bioassays. The potential role of four candidate resistance genes was assessed using qRT-PCR.
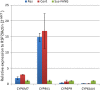
Results: An. funestus s.s. and An. parensis, were the only members of the An. funestus group identified. Both species were sympatric in Masindi (North-West), whereas only An. parensis was present in Mityana (Central) and Ntungamo (South-West). The Plasmodium falciparum infection detected in An. parensis (4.2%) by TaqMan could not be confirmed by nested PCR, whereas the 5.3% infection in An. funestus s.s. was confirmed. An. parensis was susceptible to most insecticides, however, a moderate resistance was observed against deltamethrin and DDT. In the sympatric population of Masindi, resistance was observed to pyrethroids (permethrin and deltamethrin) and DDT, but all the resistant mosquitoes belonged to An. funestus s.s. No significant over-expression was observed for the four P450 candidate genes CYP6M7, CYP9K1, CYP6P9 and CYP6AA4 between deltamethrin resistant and control An. parensis. However, when compared with the susceptible FANG An. funestus s.s strain, the CYP9K1 is significantly over-expressed in An. parensis (15-fold change; P < 0.001), suggesting it could play a role in the deltamethrin resistance.
Conclusion: The contrasting infection rates and insecticide susceptibility profiles of both species highlights the importance of accurate species identification for successful vector control programs.
Figures


References
-
- Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, Dorsey G, Kamya MR, Rosenthal PJ. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–195. doi: 10.1016/j.actatropica.2011.03.004. - DOI - PMC - PubMed
-
- Cohuet A, Simard F, Toto JC, Kengne P, Coetzee M, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med Hyg. 2003;69:200–205. - PubMed
-
- Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region) Johannesburg: South African Institute for Medical Research; 1987.
-
- Gillies MT, De Meillon B. The anophelinae of Africa South of the Sahara. Johannesburg: The South African Institute for Medical Research; 1968.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

