Building a common pediatric research terminology for accelerating child health research
- PMID: 24534404
- PMCID: PMC3934328
- DOI: 10.1542/peds.2013-1504
Building a common pediatric research terminology for accelerating child health research
Abstract
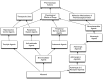
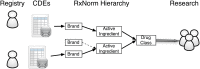
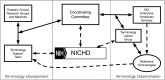
Longitudinal observational clinical data on pediatric patients in electronic format is becoming widely available. A new era of multi-institutional data networks that study pediatric diseases and outcomes across disparate health delivery models and care settings are also enabling an innovative collaborative rapid improvement paradigm called the Learning Health System. However, the potential alignment of routine clinical care, observational clinical research, pragmatic clinical trials, and health systems improvement requires a data infrastructure capable of combining information from systems and workflows that historically have been isolated from each other. Removing barriers to integrating and reusing data collected in different settings will permit new opportunities to develop a more complete picture of a patient's care and to leverage data from related research studies. One key barrier is the lack of a common terminology that provides uniform definitions and descriptions of clinical observations and data. A well-characterized terminology ensures a common meaning and supports data reuse and integration. A common terminology allows studies to build upon previous findings and to reuse data collection tools and data management processes. We present the current state of terminology harmonization and describe a governance structure and mechanism for coordinating the development of a common pediatric research terminology that links to clinical terminologies and can be used to align existing terminologies. By reducing the barriers between clinical care and clinical research, a Learning Health System can leverage and reuse not only its own data resources but also broader extant data resources.
Keywords: clinical research; governance; learning health system; ontology; pediatric research; terminology harmonization.
Figures
References
-
- Pace WD, Cifuentes M, Valuck RJ, Staton EW, Brandt EC, West DR. An electronic practice-based network for observational comparative effectiveness research. Ann Intern Med. 2009;151(5):338–340 - PubMed
-
- Maro JC, Platt R, Holmes JH, et al. Design of a national distributed health data network. Ann Intern Med. 2009;151(5):341–344 - PubMed
-
- Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care. 2010;48(suppl 6):S45–S51 - PubMed
-
- Kantak AD, Grow JL, Ohlinger J, Adams HJ, Knupp AM, Lavin JP, Jr. Management of high-order multiple births: application of lessons learned because of participation in Vermont Oxford Network collaboratives. Pediatrics. 2006;118(suppl 2):S159–S168 - PubMed
-
- Payne NR, Finkelstein MJ, Liu M, Kaempf JW, Sharek PJ, Olsen S. NICU practices and outcomes associated with 9 years of quality improvement collaboratives. Pediatrics. 2010;125(3):437–446 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

