The role of protein dynamics in GPCR function: insights from the β2AR and rhodopsin
- PMID: 24534489
- PMCID: PMC3986065
- DOI: 10.1016/j.ceb.2014.01.008
The role of protein dynamics in GPCR function: insights from the β2AR and rhodopsin
Abstract
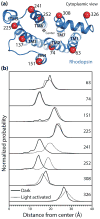
G protein-coupled receptors (GPCRs) are versatile signaling proteins that mediate complex cellular responses to hormones and neurotransmitters. Recent advances in GPCR crystallography have provided inactive and active state structures for rhodopsin and the β2 adrenergic receptor (β2AR). Although these structures suggest a two-state 'on-off' mechanism of receptor activation, other biophysical studies and observed signaling versatility suggest that GPCRs are highly dynamic and exist in a multitude of functionally distinct conformations. To fully understand how GPCRs work, we must characterize these conformations and determine how ligands affect their energetics and rates of interconversion. This brief review will compare and contrast the dynamic properties of rhodopsin and β2AR that shed light on the role of structural dynamics in their distinct signaling behaviors.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Insel PA, Head BP, Ostrom RS, Patel HH, Swaney JS, Tang CM, Roth DM. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann N Y Acad Sci. 2005;1047:166–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

