Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists
- PMID: 24534492
- PMCID: PMC3989064
- DOI: 10.1016/j.ejphar.2014.02.007
Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists
Abstract
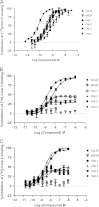
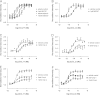
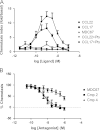
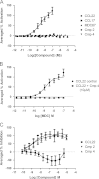
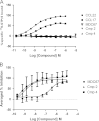
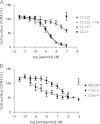
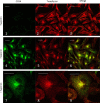
The chemokine receptor CCR4 has at least two natural agonist ligands, MDC (CCL22) and TARC (CCL17) which bind to the same orthosteric site with a similar affinity. Both ligands are known to evoke chemotaxis of CCR4-bearing T cells and also elicit CCR4 receptor internalization. A series of small molecule allosteric antagonists have been described which displace the agonist ligand, and inhibit chemotaxis. The aim of this study was to determine which cellular coupling pathways are involved in internalization, and if antagonists binding to the CCR4 receptor could themselves evoke receptor internalization. CCL22 binding coupled CCR4 efficiently to β-arrestin and stimulated GTPγS binding however CCL17 did not couple to β-arrestin and only partially stimulated GTPγS binding. CCL22 potently induced internalization of almost all cell surface CCR4, while CCL17 showed only weak effects. We describe four small molecule antagonists that were demonstrated to bind to two distinct allosteric sites on the CCR4 receptor, and while both classes inhibited agonist ligand binding and chemotaxis, one of the allosteric sites also evoked receptor internalization. Furthermore, we also characterize an N-terminally truncated version of CCL22 which acts as a competitive antagonist at the orthosteric site, and surprisingly also evokes receptor internalization without demonstrating any agonist activity. Collectively this study demonstrates that orthosteric and allosteric antagonists of the CCR4 receptor are capable of evoking receptor internalization, providing a novel strategy for drug discovery against this class of target.
Keywords: CCL17; CCL22; CCR4; Chemokine; MDC; TARC.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Andrews G., Jones C., Wreggett K.A. An intracellular allosteric site for a specific class of antagonists of the CC chemokine G protein-coupled receptors CCR4 and CCR5. Mol. Pharmacol. 2008;73:855–867. - PubMed
-
- Bonecchi R., Bianchi G., Bordignon P.P., D׳Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. - PMC - PubMed
-
- Cahn A., Hodgson S., Wilson R., Robertson. J., Watson J., Beerahee M., Hughes S.C., Young G., Graves R., Hall D., van Marle S., Solari R. Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: results from an open-label and from a randomised study. BMC Pharmacol. Toxicol. 2013;14:14. - PMC - PubMed
-
- Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P., Andrew D.P., Warnke R., Ruffing N., Kassam N., Wu L., Butcher. E.C. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. - PubMed
-
- Cheng Y., Prusoff W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

