Mutations in GNAL: a novel cause of craniocervical dystonia
- PMID: 24535567
- PMCID: PMC4237020
- DOI: 10.1001/jamaneurol.2013.4677
Mutations in GNAL: a novel cause of craniocervical dystonia
Abstract
Importance: Mutations in the GNAL gene have recently been shown to cause primary torsion dystonia. The GNAL-encoded protein (Gαolf) is important for dopamine D1 receptor function and odorant signal transduction. We sequenced all 12 exons of GNAL in 461 patients from Germany, Serbia, and Japan, including 318 patients with dystonia (190 with cervical dystonia), 51 with hyposmia and Parkinson disease, and 92 with tardive dyskinesia or acute dystonic reactions.
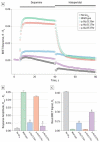
Observations: We identified the following two novel heterozygous putative mutations in GNAL: p.Gly213Ser in a German patient and p.Ala353Thr in a Japanese patient. These variants were predicted to be pathogenic in silico, were absent in ethnically matched control individuals, and impaired Gαolf coupling to D1 receptors in a bioluminescence energy transfer (BRET) assay. Two additional variants appeared to be benign because they behaved like wild-type samples in the BRET assay (p.Ala311Thr) or were detected in ethnically matched controls (p.Thr92Ala). Both patients with likely pathogenic mutations had craniocervical dystonia with onset in the fifth decade of life. No pathogenic mutations were detected in the patients with hyposmia and Parkinson disease, tardive dyskinesias, or acute dystonic reactions.
Conclusions and relevance: Mutations in GNAL can cause craniocervical dystonia in different ethnicities. The BRET assay may be a useful tool to support the pathogenicity of identified variants in the GNAL gene.
Figures

Comment in
-
GNAL mutations and dystonia.JAMA Neurol. 2014 Aug;71(8):1052-3. doi: 10.1001/jamaneurol.2014.1506. JAMA Neurol. 2014. PMID: 25111208 No abstract available.
-
GNAL mutations and dystonia--reply.JAMA Neurol. 2014 Aug;71(8):1053-4. doi: 10.1001/jamaneurol.2014.1509. JAMA Neurol. 2014. PMID: 25111209 No abstract available.
References
-
- Nasir J, Frima N, Pickard B, Malloy MP, Zhan L, Grünewald R. Unbalanced whole arm translocation resulting in loss of 18p in dystonia. Mov Disord. 2006;21(6):859–863. - PubMed
-
- Corvol JC, Studler JM, Schonn JS, Girault JA, Hervé D. Gαolf is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76(5):1585–1588. - PubMed
-
- Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244(4906):790–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

