Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner
- PMID: 24535669
- PMCID: PMC3976123
- DOI: 10.3892/ijmm.2014.1658
Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner
Abstract
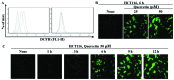
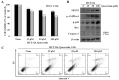
The induction of apoptosis in cancer cells is a therapeutic strategy for the treatment of cancer. In the present study, we investigated the regulatory mechanisms responsible for quercetin-induced apoptosis, mamely the increased expression of sestrin 2 and the activation of the 5' AMP-activated protein kinase (AMPK)/p38 MAPK signaling pathway. Our results revealed that quercetin induced apoptosis by generating the production of intracellular reactive oxygen species (ROS) and increasing the expression of sestrin 2. The induction of apoptosis by quercetin occurred through the activation of the AMPK/p38 signaling pathway and was dependent on sestrin 2. However, the silencing of sestrin 2 using small interfering RNA (siRNA) targeting sestrin 2 revealed that quercetin did not regulate AMPK or p38 phosphorylation in the cells in which sestrin 2 was silenced. On the other hand, it has been previously reported that sestrin 2 expression is not dependent on p53 expression under hypoxic conditions, whereas DNA damage is dependent on p53. We demonstrate that the increase in the expression of sestrin 2 by quercetin-generated intracellular ROS is p53-independent. The increased expression of sestrin 2 induced apoptosis through the AMPK/p38 signaling pathway in the HT-29 colon cancer cells, which are p53 mutant, treated with quercetin. Thus, our data suggest that quercetin induces apoptosis by reducing mitochondrial membrane potential, generating intracellular ROS production and increasing sestrin 2 expression through the AMPK/p38 pathway. In addition, p53 is not a necessary element for an apoptotic event induced by sestrin 2.
Figures




References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. - PubMed
-
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–943. - PubMed
-
- Chien SY, Wu YC, Chung JG, et al. Quercetin-induced apoptosis acts through mitochondrial- and capase-2-dependent pathways in human breast cancer MDA-MB-231 cells. Hum Exp Toxicol. 2009;28:493–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

