Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: first-in-human studies
- PMID: 24535888
- PMCID: PMC4037587
- DOI: 10.1007/s40262-014-0136-3
Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: first-in-human studies
Abstract
Background and objectives: Fexinidazole is a 5-nitroimidazole recently included in a clinical efficacy trial as an oral drug for the treatment of human African trypanosomiasis (HAT). Preclinical studies showed it acts as a pharmacologically active pro-drug with two key active metabolites: sulfoxide and sulfone (the most active metabolite). The present studies aimed to determine the best dose regimen for the treatment of stage 2 sleeping sickness patients, which could eventually also treat stage 1 patients.
Methods: Fexinidazole was assessed in 154 healthy adult male subjects of sub-Saharan African origin. Three initial first-in-human studies and two additional studies assessed a single ascending dose and multiple ascending doses (both under fasted conditions), tablet versus suspension formulation and food effect (fasted vs. high-fat meal and field-adapted food), and multiple ascending doses with a loading dose regimen under fed conditions.
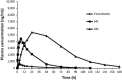
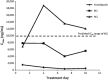
Results: Fexinidazole was well-tolerated in a single dose from 100 to 3,600 mg, with quick absorption of the parent drug and rapid metabolism into sulfoxide [time to maximum concentration (t max) 2-5 h] and sulfone (t max 18-24 h). The tablet formulation was approximately 25 % less bioavailable than the suspension, and food intake increased drug absorption and plasma concentrations of fexinidazole and its two metabolites by approximately 200 %. Fourteen-day multiple ascending dosing administered up to 3,600 mg/day in fasted conditions showed that fexinidazole was generally well-tolerated (mild to moderate, spontaneously reversible drug-related adverse events). Following the high-fat food effect finding, another study was conducted to evaluate the impact of a low-fat regimen closer to that of the target population, showing that the type of meal does not influence fexinidazole absorption. The last study showed that a loading dose of 1,800 mg/day for 4 days followed by a 1,200 mg/day regimen for 6 days with a normal meal provided the desired exposure of fexinidazole and its metabolites, particularly sulfone, with good tolerability. Based on preclinical evidence from a chronic infection mouse model, systemic drug concentrations obtained are expected to be clinically effective in stage 2 HAT.
Conclusions: These studies show that fexinidazole can be safely assessed in patients as a potential oral cure for both stages of HAT.
Figures


References
-
- Joubert J. Report of a WHO meeting on elimination of African trypanosomiasis (Trypanosoma brucei gambiense). Geneva, 3–5 December 2012. Geneva: WHO; 2013. p. 1754–824.
-
- World Health Organization. Control and surveillance of human African trypanosomiasis: report of a WHO expert committee. WHO Technical Report Series 984. Geneva: WHO; 2013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

