Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role?
- PMID: 24535935
- PMCID: PMC3925344
- DOI: 10.3390/brainsci3041445
Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role?
Abstract
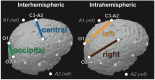
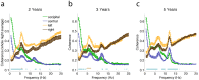
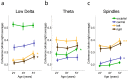
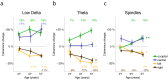
Sleep has beneficial effects on brain function and learning, which are reflected in plastic changes in the cortex. Early childhood is a time of rapid maturation in fundamental skills-e.g., language, cognitive control, working memory-that are predictive of future functioning. Little is currently known about the interactions between sleep and brain maturation during this developmental period. We propose coherent electroencephalogram (EEG) activity during sleep may provide unique insight into maturational processes of functional brain connectivity. Longitudinal sleep EEG assessments were performed in eight healthy subjects at ages 2, 3 and 5 years. Sleep EEG coherence increased across development in a region- and frequency-specific manner. Moreover, although connectivity primarily decreased intra-hemispherically across a night of sleep, an inter-hemispheric overnight increase occurred in the frequency range of slow waves (0.8-2 Hz), theta (4.8-7.8 Hz) and sleep spindles (10-14 Hz), with connectivity changes of up to 20% across a night of sleep. These findings indicate sleep EEG coherence reflects processes of brain maturation-i.e., programmed unfolding of neuronal networks-and moreover, sleep-related alterations of brain connectivity during the sensitive maturational window of early childhood.
Keywords: children; coherence; development; early childhood; maturation; sleep EEG.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
