Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer
- PMID: 24535937
- PMCID: PMC3925074
- DOI: 10.1158/2326-6066.CIR-13-0126
Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer
Abstract
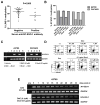
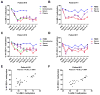
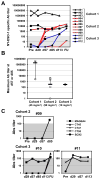
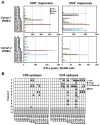
The cancer-testis/cancer-germline antigen NY-ESO-1 is a vaccine target in epithelial ovarian cancer (EOC), but its limited expression is a barrier to vaccine efficacy. As NY-ESO-1 is regulated by DNA methylation, we hypothesized that DNA methyltransferase (DNMT) inhibitors may augment NY-ESO-1 vaccine therapy. In agreement, global DNA hypomethylation in EOC was associated with the presence of circulating antibodies to NY-ESO-1. Pre-clinical studies using EOC cell lines showed that decitabine treatment enhanced both NY-ESO-1 expression and NY-ESO-1-specific CTL-mediated responses. Based on these observations, we performed a phase I dose-escalation trial of decitabine, as an addition to NY-ESO-1 vaccine and doxorubicin liposome (doxorubicin) chemotherapy, in 12 patients with relapsed EOC. The regimen was safe, with limited and clinically manageable toxicities. Both global and promoter-specific DNA hypomethylation occurred in blood and circulating DNAs, the latter of which may reflect tumor cell responses. Increased NY-ESO-1 serum antibodies and T cell responses were observed in the majority of patients, and antibody spreading to additional tumor antigens was also observed. Finally, disease stabilization or partial clinical response occurred in 6/10 evaluable patients. Based on these encouraging results, evaluation of similar combinatorial chemo-immunotherapy regimens in EOC and other tumor types is warranted.
Figures

References
-
- Storni T, Ruedl C, Renner WA, Bachmann MF. Innate immunity together with duration of antigen persistence regulate effector T cell induction. J Immunol. 2003;171:795–801. - PubMed
-
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. - PubMed
-
- Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–33. - PubMed
-
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:2346–57. - PMC - PubMed
-
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16168–73. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

