Molecular pathways: emerging roles of mammalian Sirtuin SIRT7 in cancer
- PMID: 24536059
- PMCID: PMC3980586
- DOI: 10.1158/1078-0432.CCR-13-1547
Molecular pathways: emerging roles of mammalian Sirtuin SIRT7 in cancer
Abstract
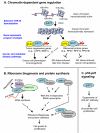
SIRT7 belongs to the Sirtuin family of NAD-dependent enzymes, the members of which play diverse roles in aging, metabolism, and disease biology. Increased SIRT7 expression is observed in human cancers and growing evidence suggests important SIRT7 functions in fundamental cellular programs with an impact on oncogenic transformation and tumor biology. SIRT7 associates with chromatin, where it catalyzes selective deacetylation of lysine 18 on histone H3 (H3K18), an emerging epigenetic biomarker of aggressive tumors and poor clinical outcome in patients with cancer. Through H3K18 deacetylation at specific promoters, SIRT7 controls a tumor-suppressive gene expression program that stabilizes the transformed state of cancer cells. SIRT7 also orchestrates several molecular processes, including rRNA and tRNA synthesis, which ultimately promote the increased ribosome biogenesis necessary for tumor cell growth and proliferation. Remarkably, inactivation of SIRT7 can reverse the transformed phenotype of cancer cells and reduce their tumorigenicity in vivo. These findings place SIRT7 at the crossroads of chromatin signaling, metabolic, and tumor-regulatory pathways. Thus, SIRT7 is a promising pharmacologic target for epigenetic cancer therapy. The development of SIRT7 modulators may allow new therapeutic strategies that control tumor progression by reprogramming the chromatin landscape and biosynthetic machinery of cancer cells.
©2014 AACR.
Figures
References
-
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

