Patient information leaflets (PILs) for UK randomised controlled trials: a feasibility study exploring whether they contain information to support decision making about trial participation
- PMID: 24548781
- PMCID: PMC3936815
- DOI: 10.1186/1745-6215-15-62
Patient information leaflets (PILs) for UK randomised controlled trials: a feasibility study exploring whether they contain information to support decision making about trial participation
Abstract
Background: Informed consent is regarded as a cornerstone of ethical healthcare research and is a requirement for most clinical research studies. Guidelines suggest that prospective randomised controlled trial (RCT) participants should understand a basic amount of key information about the RCTs they are being asked to enrol in in order to provide valid informed consent. This information is usually provided to potential participants in a patient information leaflet (PIL). There is evidence that some trial participants fail to understand key components of trial processes or rationale. As such, the existing approach to information provision for potential RCT participants may not be optimal. Decision aids have been used for a variety of treatment and screening decisions to improve knowledge, but focus more on overall decision quality, and may be helpful to those making decisions about participating in an RCT. We investigated the feasibility of using a tool to identify which items recommended for good quality decision making are present in UK PILs.
Methods: PILs were sampled from UK registered Clinical Trials Unit websites across a range of clinical areas. The evaluation tool, which is based on standards for supporting decision making, was applied to 20 PILs. Two researchers independently rated each PIL using the tool. In addition, word count and readability were assessed.
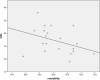
Results: PILs scored poorly on the evaluation tool with the majority of leaflets scoring less than 50%. Specifically, presenting probabilities, clarifying and expressing values and structured guidance in deliberation and communication sub-sections scored consistently poorly. Tool score was associated with word count (r=0.802, P <0.01); there was no association between score and readability (r=-0.372, P=0.106).
Conclusions: The tool was feasible to use to evaluate PILs for UK RCTs. PILs did not meet current standards of information to support good quality decision making. Writers of information leaflets could use the evaluation tool as a framework during PIL development to help ensure that items are included which promote and support more informed decisions about trial participation. Further research is required to evaluate the inclusion of such information.
Figures
References
-
- World Medical Association (WMA) WMA Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Ferney-Voltaire: WMA; 2008. http://www.wma.net/en/30publications/10policies/b3/index.html.
-
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Oxford: Oxford University Press; 1994.
-
- International Conference on Harmonisation (ICH) ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R1) Geneva: ICH; 1996. (Step 4 version).
-
- National Research Ethics Service (NRES) Information Sheet and Consent Forms: Guidance for Researchers and Reviewers. London: National Health Service, National Patient Safety Agency; 2009.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials